Chemistry Exam 4 Review
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
Convert mole of Atom to # of atoms
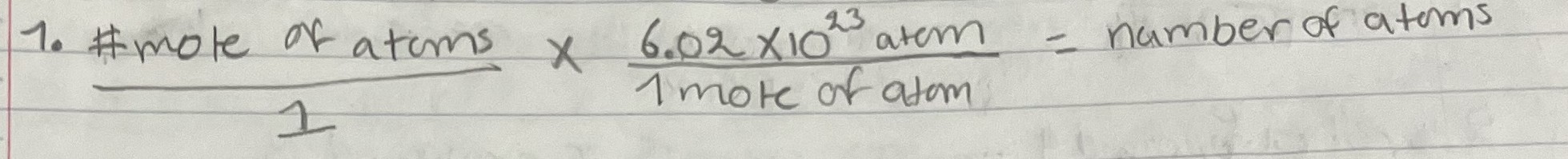
Convert mole of molecules to # of molecule convert that to one of the atom in the formula
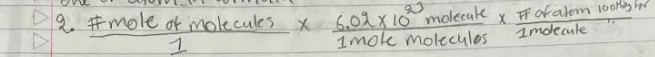
Atoms or molecule to moles
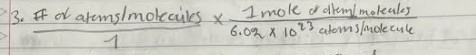
Calculate molar ass of compound
take each atoms and multiply by their subscript and add all to get g/mol
Calculate grams to mols using molar mass
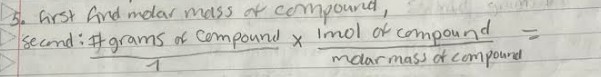
calculate mols to grams using molar mass

Convert from gras to atoms

convert from atoms to grams

Convert Mol A to Mol B

mol compound A to grams of compound B

gram compound A to mol compound b

grams compound A to grams B
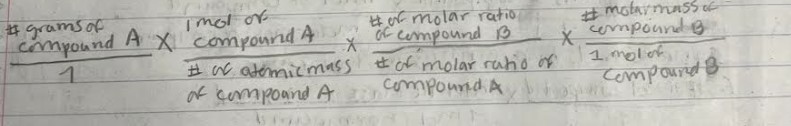
Limiting Reactant
the one that run out 1st; divide mol by their respective coefficient; the smallest
Theoretical Yield in mols
Compare the 2 amount of mol of compound C; it’s the smallest and also limiting reactant

Percent yield
(Actual yield / theoretical yield) x 100
Percent error
100 - percent yield
Excess reactant left over

What is the actual yield is associated
product
Theoretical Yield in grams

Combustion Reaction
any mixture of C with addition to O produce CO2 and H2O
Synthesis or combination
An element reacting with another to make a compound
Decomposition Reaction
breaking down complex formula
Single Replacement
pure element react with compound and pair with and of the element in the compound
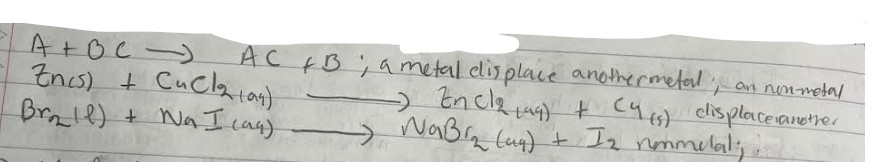
Double Replacement
a compound react with another comp

Precipitation
when 2 aqueous solution mixed from double replacement and get a solid
Acid Base Neutralization
In double replacement when two aqueous solution one acid ad one base and it result with salt + water