alkyne reactivity
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Last updated 8:13 PM on 10/15/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
1
New cards
addition of h-x
turns into alkene
markovnikov
in excess, turns into alkane (but that second part of the rxn is slower)

2
New cards
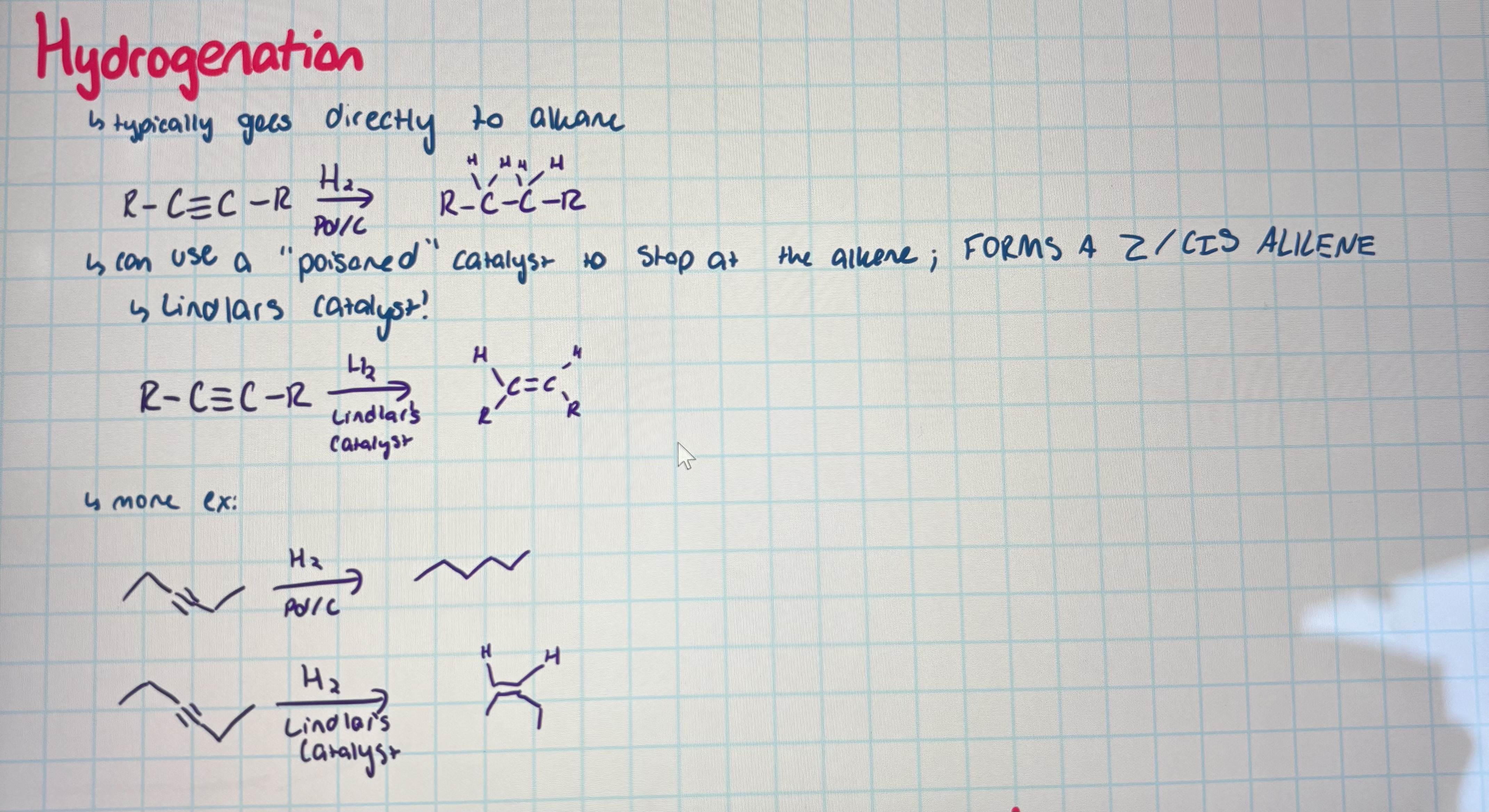
hydrogenation
adding H2 and Pd/C turns it to an alkane
using lindlars catalyst turns it into a z/cis alkene
3
New cards
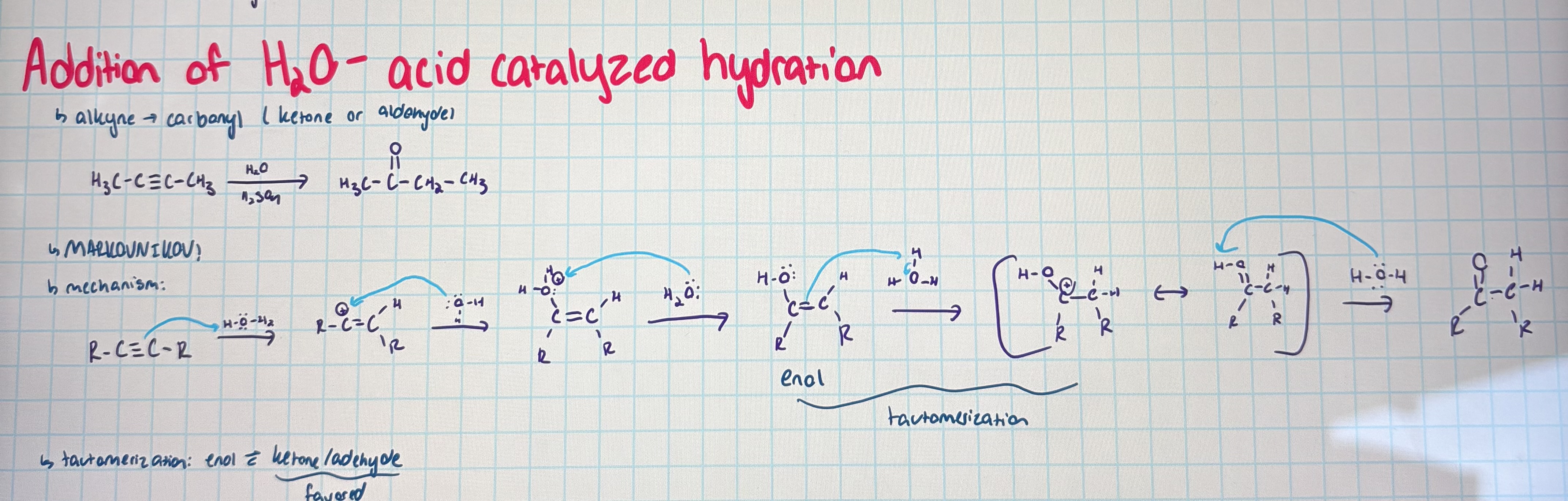
acid catalyzed hydration
markovnikov
turns alkyne into a carbonyl
tautomerization: enol in equilibrium with a carbonyl (carbonyl is favored)
4
New cards
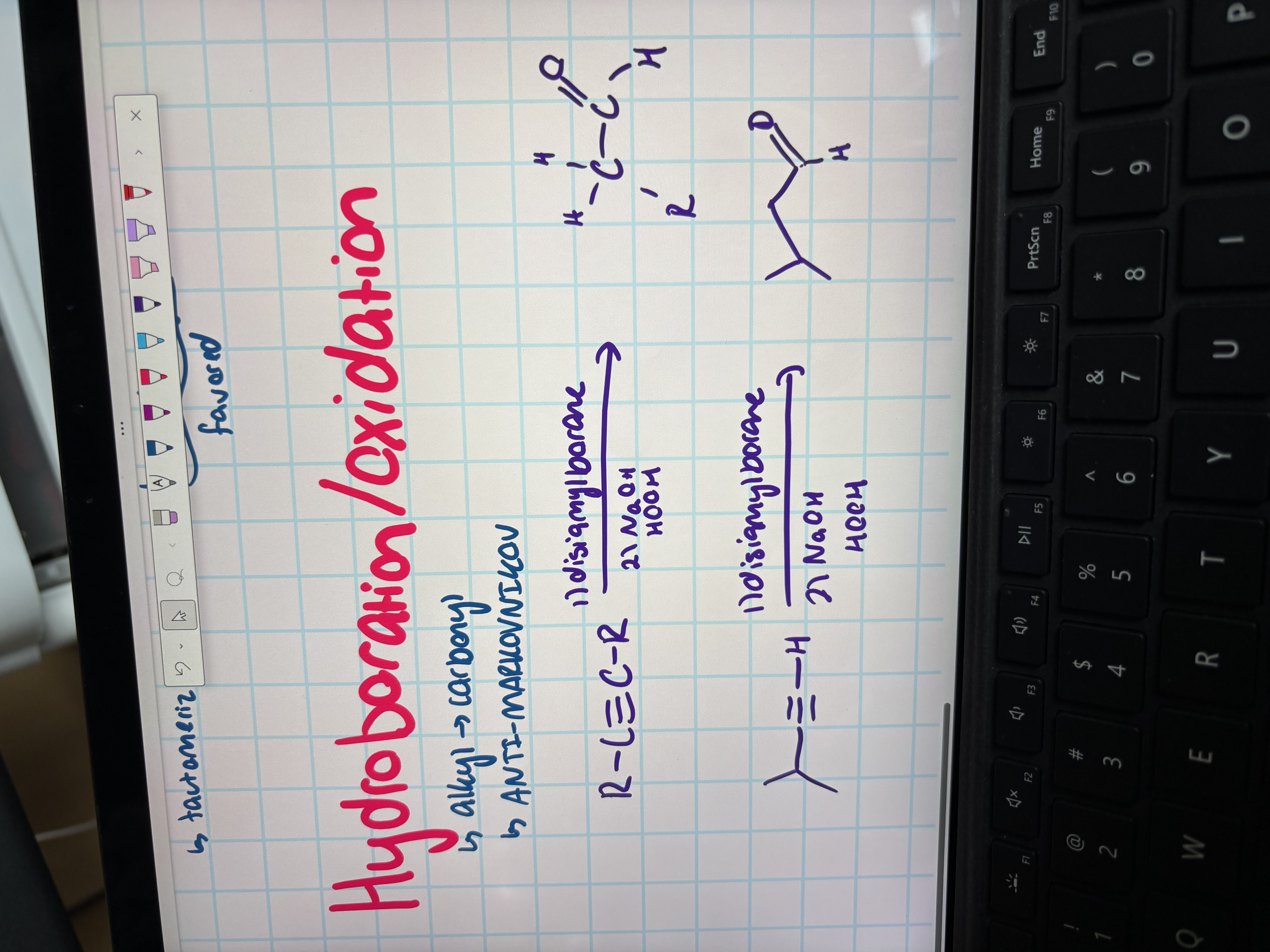
hydroboration/oxidation
alkyl to carbonyl
antimarkovnikov
used disiamylborane in step 1 then NaOH and HOOH in step 2