oxidation states and redox
1/17
Earn XP
Description and Tags
i cannot describe how to combine half equations soz
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
what is oxidation (in terms of oxygen and electrons)?
gain of O2
the process of e- loss
what is reduction (in terms of oxygen and electrons)?
loss of O2
the process of e- gain
what is an oxidising agent (in terms of oxidation and in terms of electrons)?
an agent which causes a substance to be oxidised (and so are reduced themselves)
this makes them e- acceptors
what is a reducing agent (in terms of reduction and electrons)?
an agent which causes a substance to be reduced (and so are oxidised themselves)
this makes them e- donors
what is a half equation?
an equation showing the loss or gain of e-
what is an oxidation state?
a +ve/-ve number showing how many e- an element has lost/gained
what is the oxidation state of an uncombined element?
0

what is the sum of the oxidation states in a compound?
0
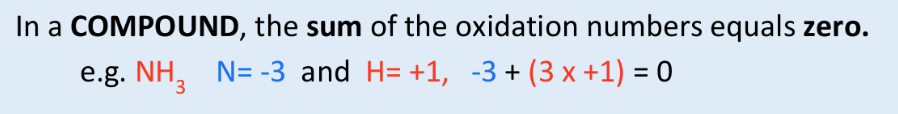
what is the sum of the oxidation numbers in an ion?
the charge on the ion

what is the oxidation state of F usually?
-1

what is O’s oxidation state usually? what are the exceptions?
-2
except when combined w/ F or in peroxides (compound where 2 O atoms are joined by a single cov bond)

what is the oxidation state of a group I metal always?
+1
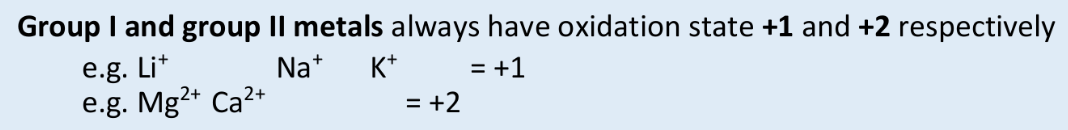
what is the oxidation state of a group II metal always?
+2
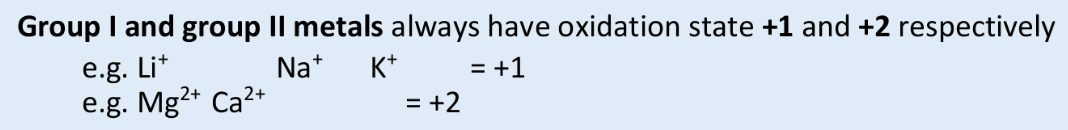
what is the oxidation state of H usually? what are the exceptions?
+1
except when combined w/ group I or II metals
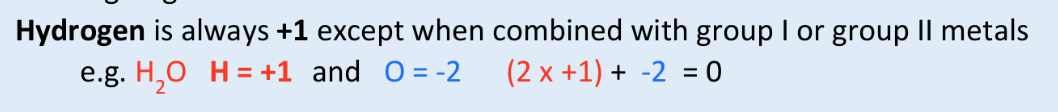
what is the oxidation state of a halide usually? what is the exception?
-1
except when combined w/ F or O

what does an increase in oxidation state mean (in terms of oxidation/reduction and e-)?
oxidation has occurred
so e- have been lost
what does a decrease in oxidation state mean (in terms of oxidation/reduction and e-)?
reduction has occurred
so e- have been gained
give the 4 steps needed to balance a half equation:
balance element changing oxidation state
balance using H2O
balance using H+
balance charge w/ e-