ELECTRONIC CONFIGURATIONS | 1.4
0.0(0)
0.0(0)
New
Card Sorting
1/9
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
1
New cards
Electronic Configuration
describes the distribution of e- among the various atomic orbitals in the ground state
2
New cards
Aufbau Principle

3
New cards
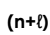
How to get the energy of the orbital?
4
New cards
Hund’s Rule of Multiplicity
all degenerate orbitals in given subshell are SINGLY occupied by electrons before pairing begins
unpaired electrons must have parallel spins
5
New cards
Electron Configuration, noble gas core configuration/short hand rule, valence electron configuration
Ways of Writing Configurations
6
New cards
Unusual Configurations

7
New cards
Cr, Cu, Mo, Ag, Au
Elements that are Unusual Configurations
8
New cards
CATIONS

9
New cards
ANIONS

10
New cards
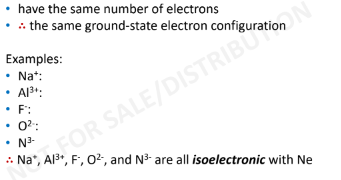
Isoelectronic