Midterm 1 Memorization + Enzyme Thermodynamics
1/78
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
79 Terms
What are the 3 methods for determining the structure of intact proteins?
cryo-electron microscopy: beam of electrons to visualize a frozen protein
nuclear magnetic resonance spectroscopy: measures the location of nuclei
x-ray crystallography: uses x-rays to measure electron density
list the 7 post translational modifications of proteins for bioc 202
phosphorylation
glycosylation
hydroxylation
carboxylation
acetylation
methylation
cleavage
what is phosphorylation and which amino acid residue(s) is it commonly associated with?
phosphate group attaches to -OH
activates or inactivates a protein
associated with: (aa with -OH part of its R group)
Serine, Threonine, Tyrosine
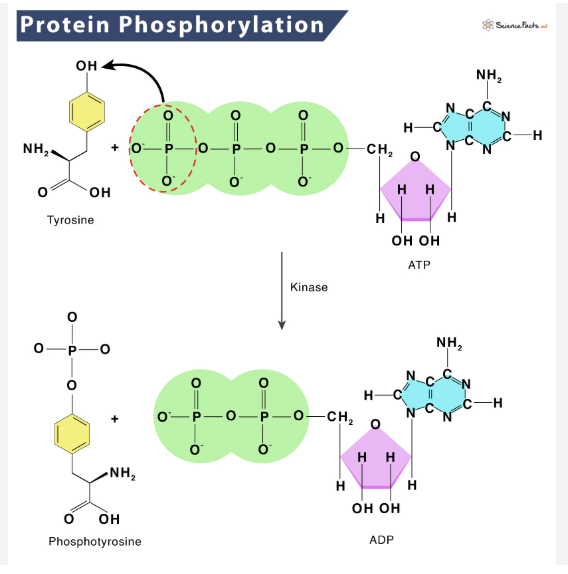
What is glycosylation and which amino acid residue(s) is it commonly associated with?
attachment of one or more sugars to a residue
purposes include surface labelling (most proteins on the cell surface are glycosylated)
associated with: Asparagine, Threonine, Serine and Glutamine (all polar/hydrophillic)
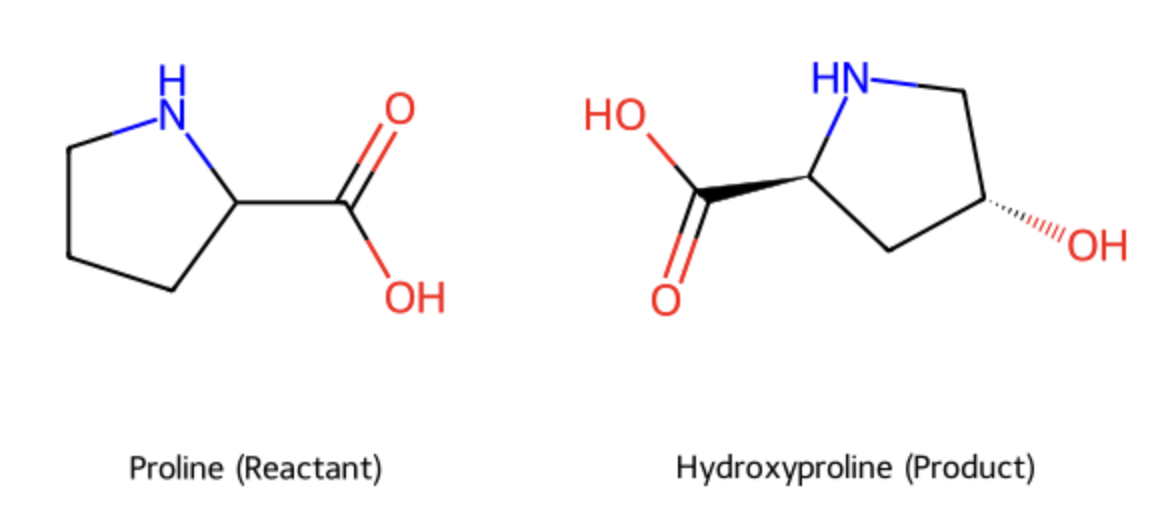
what is hydroxylation and which amino acid residue(s) is it commonly associated with?
addition of an OH (hydroxyl group)
fibre stabilization: vital for collegen
associated with: Proline
what is carboxylation and which amino acid residue(s) is it commonly associated with?
addition of a carboxyl group
usually to Glutamate
clotting
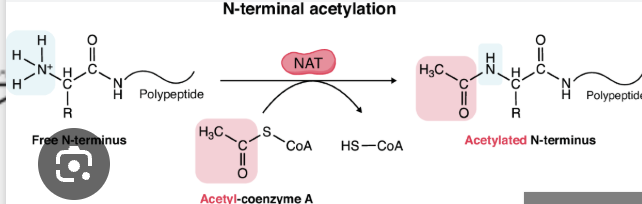
what is acetylation and which amino acid residue(s) is it commonly associated with?
addition of an acetyl group to an NH3+ (amino group)
associated with Lysine
what is methylation and which amino acid residue(s) is it commonly associated with?
the addition of a methyl group to electronegative atoms (N, O)
associated with Arginine, Lysine, Glutamate, Aspartate
what is cleavage and which amino acid residue(s) is it commonly associated with?
most proteins are trimmed after synthesis to activate or inactivate them
multiple proteins from one polypeptide is common
enzyme
biological macromolecule (large molecule; carbohydrates, lipids, proteins, nucleic acids), usually a protein, that acts as a catalyst for biological reactions
highly specific and only catalyze a particular set of reactions
catalyst
a substance that increases the rate of a chemical reaction without itself undergoing any permanent change
they do this by lowering activation energy
kinetics
the branch of chemistry concerned with the rates of chemical reactions
enzyme kinetics studies how enzymes bind to their substrates and turn them into products
substrate
a molecule that an enzyme acts on
the substrate binds to the enzyme’s active site through a series of weak interactions
apoenzyme
an enzyme that is inactive because it is missing its cofactor (a required non-protein component)
cofactor
an inorganic ion or a small organic compound required for an enzyme’s activity
holoenzyme
a complete, catalytically active enzyme, consisting of an apoenzyme combined with its necessary cofactor(s)
prosthetic group
a cofactor that is tightly or covalently bound to the active site of an enzyme. A heme group is a common example
transition state
(X‡)
a temporary high energy and unstable state that a substrate must pass through to be converted into a product. It is the point of highest free energy in the chemical reaction (because old chemical bonds are in the process of breaking and new ones are just beginning to form)
also has the lowest concentration
how exactly does an enzyme catalysis work?
the active site of an enzyme acts as a perfect tool for the high-energy unstable “in-between” molecule that exists at the transition state
the enzyme’s active site fits the transition state in two critical ways:
shape: the 3D shape of the active site pocket is precisely arranged to fit the specific bond angles and shape of the transition state molecule
charge: the arrangement of amino acids within the active site creates a specific chemical environment to stabilize it
*by being perfectly suited to hold and stabilize this unstable transition state, the enzyme drastically reduces the amount of energy required to form it, therby reducing the activation energy and speeding up the reaction
activation energy
(ΔG‡)
the minimum amount of energy required for reactants to transform into the transition state
enzymes speed up reactions by lowering this energy barrier
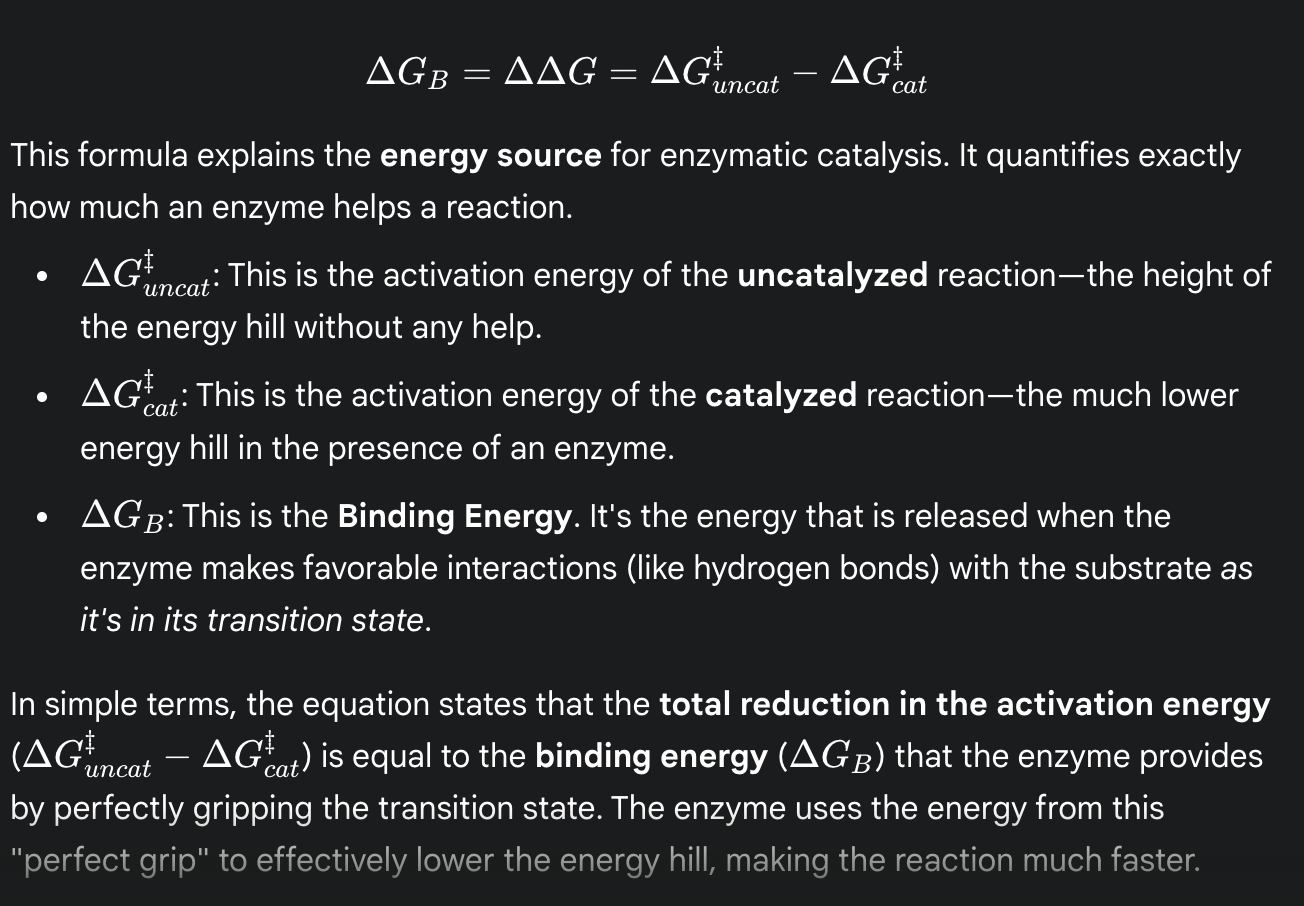
binding energy
(ΔGʙ)
the energy released when an enzyme binds to and stabilizes the transition state
this energy is the primary source for lowering the activation energy
rate of reaction (V, velocity)
The speed at which a reaction proceeds, typically measured as the concentration of product formed per unit of time. Scientists use the initial velocity because at this point, the substrate concentration is at its highest and there is virtually no product yet, which simplifies the measurement and prevents other factors (like the reverse reaction) from influencing the rate.
Rate Constant (k)
a proportionality constant that relates the rate of a reaction to the concentration of the reactants
V0
initial velocity
the reaction rate measured at the beginning of the reaction, when the concentration of the product is negligible
Michaelis-Menten Equation
What It Is: The Michaelis-Menten equation is a mathematical formula that describes the speed (velocity) of a reaction catalyzed by an enzyme. It connects the initial reaction rate (V₀) to the concentration of the substrate ([S]). This allows us to predict how fast an enzyme will work under different conditions.
Where:
V₀ is the initial rate of the reaction.
Vₘₐₓ is the maximum possible rate when the enzyme is completely saturated with substrate.
[S] is the concentration of the substrate.
Kₘ is the Michaelis constant, representing the substrate concentration at which the reaction rate is half of Vₘₐₓ.
Key Shortcut: A crucial concept for solving problems quickly is the definition of Kₘ. If you are told that the reaction is proceeding at exactly half of its maximum speed (V₀ = ½Vₘₐₓ), you immediately know that the substrate concentration must be equal to the Kₘ value ([S] = Kₘ).
![<p><strong>What It Is</strong>: The Michaelis-Menten equation is a mathematical formula that describes the speed (velocity) of a reaction catalyzed by an enzyme. It connects the initial reaction rate (V₀) to the concentration of the substrate ([S]). This allows us to predict how fast an enzyme will work under different conditions.</p><p></p><p>Where:</p><ul><li><p><strong>V₀</strong> is the initial rate of the reaction.</p></li><li><p><strong>Vₘₐₓ</strong> is the maximum possible rate when the enzyme is completely saturated with substrate.</p></li><li><p><strong>[S]</strong> is the concentration of the substrate.</p></li><li><p><strong>Kₘ</strong> is the Michaelis constant, representing the substrate concentration at which the reaction rate is half of Vₘₐₓ.</p></li></ul><p></p><p><strong>Key Shortcut</strong>: A crucial concept for solving problems quickly is the definition of Kₘ. If you are told that the reaction is proceeding at exactly half of its maximum speed (V₀ = ½Vₘₐₓ), you immediately know that the substrate concentration must be equal to the Kₘ value ([S] = Kₘ).</p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/c43256f1-ed65-4b9e-aeb6-831cc1d5ad85.png)
Michaelis Constant (Km)
The substrate concentration required for the enzyme to reach half of its maximum velocity (½ Vₘₐₓ). The Kₘ value is a crucial indicator of an enzyme's affinity for its substrate.
Low Kₘ: Indicates high affinity. The enzyme can work efficiently even at low substrate concentrations.
High Kₘ: Indicates low affinity. The enzyme needs a lot of substrate to be present to work efficiently.
Vmax
The absolute fastest rate at which an enzyme can catalyze a reaction. This speed limit is reached when the enzyme is fully saturated with substrate—meaning every single active site on every enzyme molecule is occupied and working. Adding more substrate at this point will not make the reaction go any faster.
kcat (turnover number)
The maximum number of substrate molecules a single enzyme active site can convert into product per unit of time. It is the most direct measure of an enzyme's catalytic efficiency and represents its intrinsic top speed when fully saturated.
irreversible inhibition
occurs when an inhibitor binds to an enzyme, often covalently and permantely deactivates it.
aspirin for example, irreversibly inhibits the cyclooxyrgenase (COX) enzyme
reversible inhibition
occurs when an inhibitor binds non-covalently to an enzyme, and the enzyme-inhibitor complex can dissociate
this type of inhibition can be overcome
competitive inhibition
a type of reversible inhibition where the inhibitor resembles the substrate and competes for the same active site.
non-competitive inhibition
a type of reversible inhibition where the inhibitor binds to a different site on the enzyme (an allosteric site), changing the enzyme's shape and reducing its activity.
alanine for example acts as a non-competitive inhibitor for the enyme pyruvate kinase
lineweaver-burk plot
a graphical representation of enzyme kinetics, obtained by plotting the reciprocal of the initial velocity (1/V0) against the reciprocal of the substrate concentration. It linearizes the michaelis-menten equation making it easier to determin Vmax and Km
what are the 3 examples of enzymes introduced and what do they do?
papain: speeds up the cleaving of any peptide bond
trypsin: speeds up the cleaving of only the peptide bond on the carboxyl end of Lysine and Arginine
thrombin: speeds up the cleaving of only Arginine-Glycine peptide bonds
characteristics of the active site
they are clefts (aka dimples on the enzyme made up of residues from all over the active site)
occupy a small volume of the enzyme
water is excluded or manipulated from the active site
the substrate is bound to the active site by a series of weak interactions
what is required for an enzyme to function?
it must be partly complementary to its substrate
the general 3D shape of the substrate must match the shape of the active site and the chemical properties (pos, neg, oily, watery region) of the substrate must align with the corresponding properties in the active site
BUT not perfectly…
the substrate and enzyme need a good enough initial fit to bind to each other but the initial binding causes the enzyme to slightly change. its shape, clamping down or wrapping around the substrate more tightly
this creates a more precise and stable connection, perfectly positioning the substrate for the chemical reaction to occur
explain and connect the lock and key and induced binding models
The Lock and Key model is the original, simpler idea. It proposes that the enzyme's active site is a rigid structure that is already a perfect geometric and chemical match for its specific substrate, just like a key fits into its one specific lock.
The Induced Fit model is the modern and more accurate refinement. It states that the enzyme's active site is flexible, not rigid. The initial binding of the substrate is good but not perfect, and this interaction induces the active site to change its shape to wrap more tightly around the substrate.
The connection is that the Induced Fit model builds upon and improves the Lock and Key concept. It keeps the essential idea of a specific match but adds the crucial element of enzyme flexibility. This flexibility is what allows the enzyme to stabilize the reaction's transition state, which is the key to speeding up the reaction.
do enzymes alter the final equilibrium of products to reactants or alter the change of free energy of the reaction?
no
can enzymes change the spontaneity of a reaction, for example make a non-spontaneous reaction with positive change in gibbs free energy spontaneous?
no
is the active site of the enzyme perfectly complementary to the transition state?
yes
what do the following symbols represent? GS, GP, GX‡
GS is the starting energy level of the Substrate.
GP is the final energy level of the Product.
GX‡ is the energy of the Transition State—the highest-energy, most unstable point in the middle of the reaction.
what is the formula for the activation energy for the forward reaction
(the amount of energy needed to get from the starting energy level of the substrate up to the peak of the energy hill)
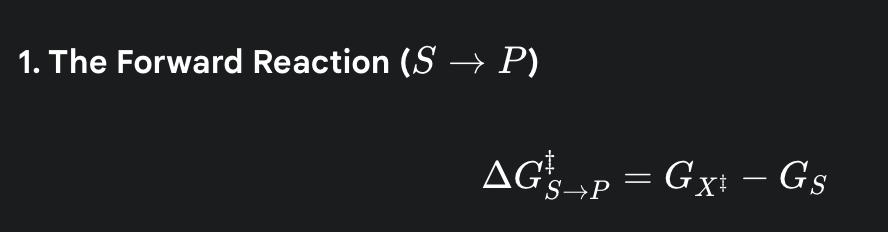
what is the formula for the activation energy for the reverse reaction
(the amount of energy required to get the energy level of the product back up to the same energy hill)
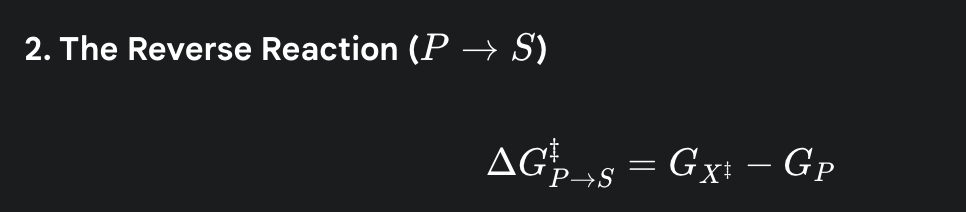
reaction pathway (physical steps of an enzyme reaction)

formula for binding energy
(the total reduction in activation energy is equal to the binding energy that the enzyme provides by perfectly gripping the transition state)
what bond joins amino acids
peptide bonds
what is phi and psi angles?
phi: dihedral angles of N-Ca
psi: dihedral angles Ca-C=O
what are the characteristics of the bonds in a polypeptide chain
C=O to NH bond is locked due to double bond character as a result of resonance; results in same plane
psi and phi bonds (other bonds beside 1) have free rotation
BUT not all combinations of psi and phi angles are possible (combinations defined by ramachandran plot)
what are the psi and phi angles for alpha helices
phi = -60 degrees
psi = -45 degrees
(minimizes steric hinderance and allows for H-bonding)
what is the intrachain H bonding pattern for alpha helices
i (C=O) binds with i + 4 (N-H)
all C=O and N-H bind except for the ends
how are the R groups arranged in alpha helices
all perpendicular the backbone (R groups stick out)
how is the helix rise affected per amino acid residue added
1.5 A (angstrom) per aa residue
what is the pattern for R groups that face the same direction (interact with the same molec) for alpha helices
i, i + 3, i + 4
this is because R groups are about 100 degrees apart (a full round is 360 degrees)
i (0), i + 3 (300), i + 4 (400)
what are psi and phi for anti-parallel beta sheets
phi: -139
psi: +135
what are phi and psi for parallel beta sheets
phi: - 119
psi: +113
what is the H bonding pattern for antiparallel beta sheets
linear
i -- j
C=O on aa i bonds with N-H on aa j
what is the H bonding pattern for parallel beta sheets
C = O i bonds with N-H j + 2
N - H i bonds with C = O j
less stable, angled bonding
how is the length of antiparallel and parallel beta sheets affected by the addition of an amino acid residue
antiparallel: + 3.5 A per aa residue
parallel: + 3.25 A per aa residue
psi and phi dihedral angles for tertiary structure
aligns with ramachandran plot
acidic aa + R groups
aspartate: 1 c, carboxyl
glutamate: 2c carboxyl
basic aa + R groups
histidine: 1 c, imidazole (5 mem, N and NH)
arginine: 3c, guanidinium
lysine: 4c, amino
special aa + side chains
cysteine: 1c, thiol (disulfide bonding)
selenocysteine: 1c, SeH
proline: 5 mem w/ amino (NP), R config
Glycine: NONE (NP) (achiral)
polar aa + R groups
Asparagine: 1 c, amide
Glutamine: 2 c, amide
Threonine: 2 c, OH branch
Serine: 1 c , OH
hydrophobic (mostly np) aa + R groups
Alanine: 1 c
Valine: 2 c, 1 c branch
Isoleucine: 3 c, 1 c branch (1)
Leucine: 3 c, 1 c branch (2)
Methionine: 2 c, S, 1 c
Phenylalamine: 1 c, phenyl
Tyrosine: 1 c, phenyl, OH (opp starting c)
Tryptophan: 1 c, indole (5 mem NH (3), 6 mem)
which aa have R groups with no carbon chains
glycine and proline
which aa have R groups with 1 carbon chains
cys (2), rings (4), asp (2), alanine + serine
cysteine
selenocysteine
phenylalamine
tyrosine
tryptophan
histidine
asparagine
aspartate
alanine
serine
which aa have R groups with 2 carbon chains
Glu, MVP
Glutamine
Glutamate
Methionine
Valine
Threonine (Polar)
which aa have R groups with 3 carbon chains
Leu (2), Arg
Leucine
Isoleucine
Arginine
which aa has a R group with a 4 carbon chain
Lysine
What are the amino acids for the following 1 letter codes:
R, N, D, Q, E, K, U, Y, W, F, T, A
Arginine, Asparagine, Aspartate, Glutamine, Glutamate, Lysine, Selenocysteine, Tyrosine, Tryptophan, Phenylalamine, Threonine, Alanine
what are the 3 letter codes for the following amino acids :
Glutamate, Aspartate, Glutamine, Asparagine, Isoleucine, Tryptophan, Selenocysteine
Glu, Asp, Gln, Asn, Ile, Trp, Sec
what is the exception to favouring trans peptide bonds
X-Proline
some can be cis bc both have steric hinderance (the energy diff between cis and trans is smaller compared to the diff w other aa)
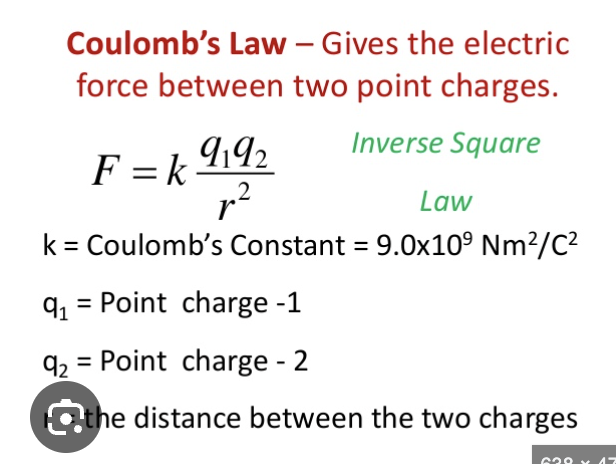
coulomb’s constant
proportionality constant (depends on dielectric constant); converts to the correct units to end up with Newtons
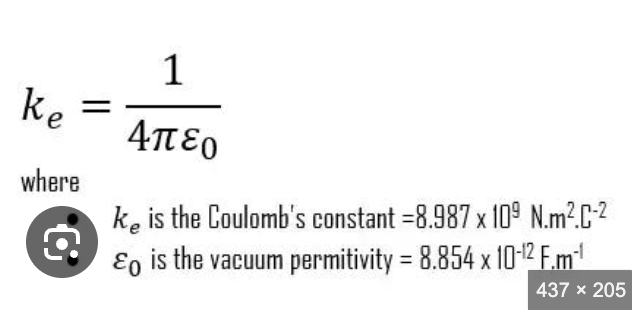
coulomb’s law
pKa
specific pH at which a functional group is exactly 50% protonated and 50% deprotonated (tipping point)
buffering zone is when pKa is within ± 1 of the pH (a certain amount of acid/base needs to be added to overcome this
what are the functional groups among the amino acids
hydrophobic
alkyl (just carbon; alanine, valine, isoleucine, leucine)
phenyl (phenylalamine)
phenol (tyrosine)
indole (tryptophan; 5 mem ring, NH (3) + 6 mem)
thio-ether (methionine: c - s - c)
pyrrolidine (proline)
hydrophilic
hydroxyl (serine, threonine)
amide (asparagine, glutamine)
thiol (cysteine; can form disulfide bonds, weakly H bonds)
SeH
basic (neutral to +)
imidazole (histidine; 5 mem, N (2), NH (3)
guanidinium (arginine; NH, NH, NH2)
side-chain amino (lysine)
acidic (neutral to -)
side chain carboxyl (aspartate, glutamate)
which aa has constrained phi rotation?
proline
strongest interaction among aa
cysteine - cysteine
disulfide bond (covalent)
a-helix wreckers
proline + glycine