CHEM-107 - Ch.11 formative
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
41 Terms
A formula that shows the arrangement of all bonds in a molecule is called a(n)...
expanded structural formula
A hydrocarbon contains only the elements ...
carbon and hydrogen
Which of the following is not typical of most hydrocarbons?
high melting point
3 multiple choice options
An organic compound composed of hydrogen and carbon connected only by single bonds is an ...
alkane
Which of these formulas is the expanded structural formula for an alkane with three carbon atoms?
H H H
H-C-C-C-H
H H H
3 multiple choice options
What is the IUPAC name of the continuous chain alkane with six carbons?
Hexane
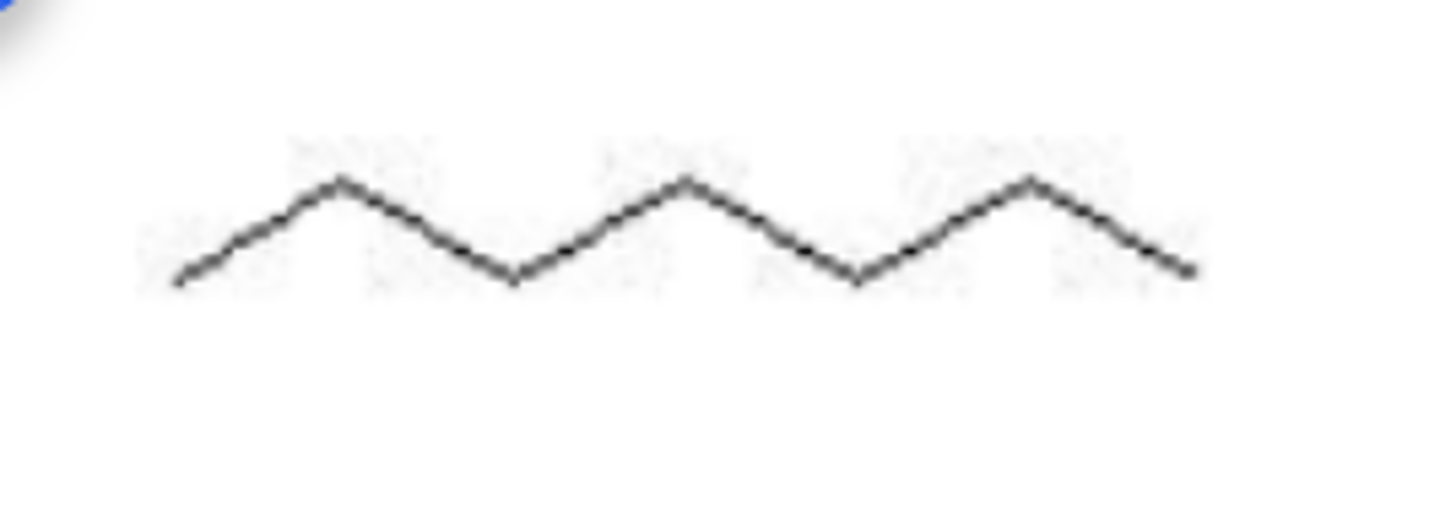
The skeletal formula shown is that of
Heptane
3 multiple choice options
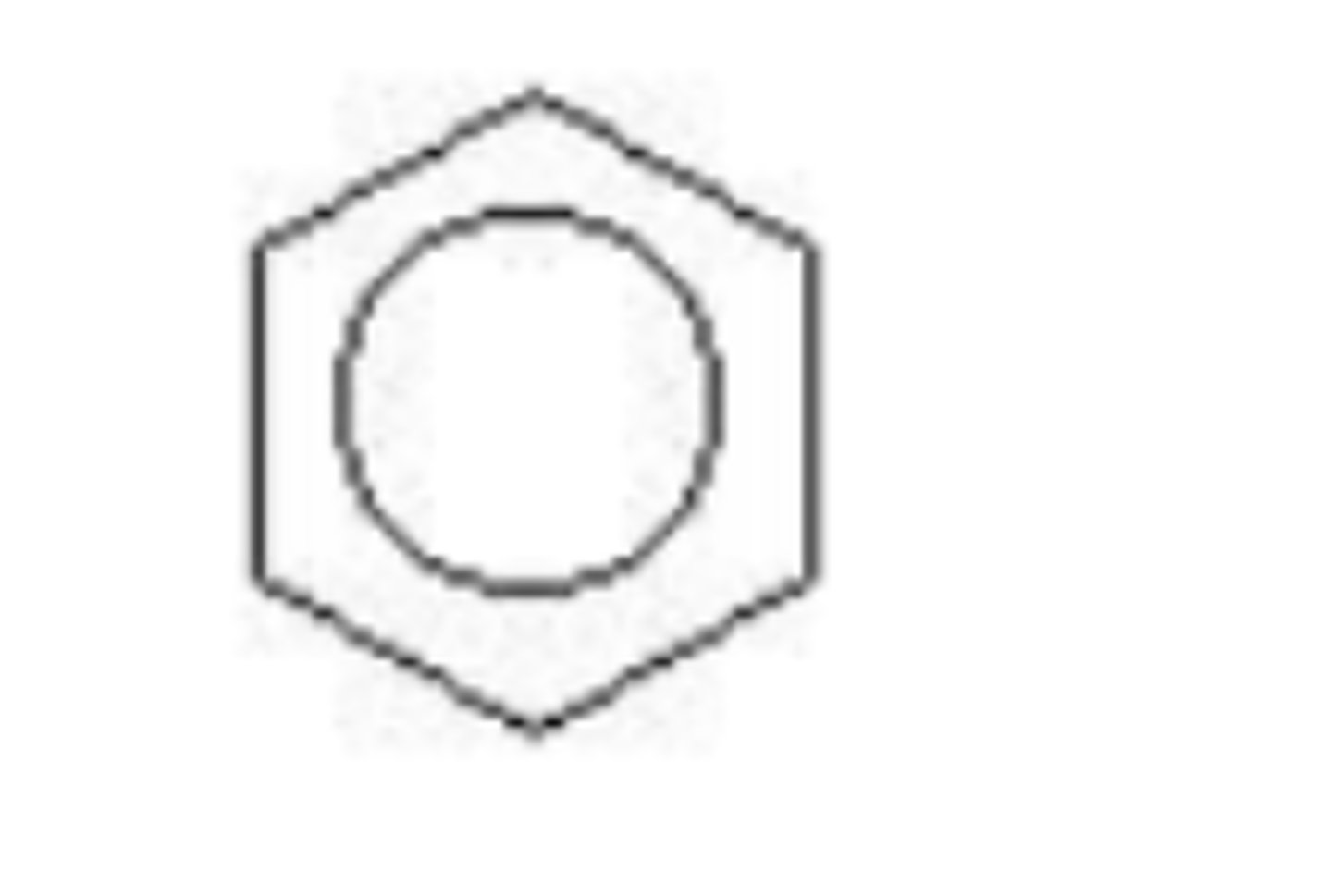
A cycloalkane ____
has two fewer hydrogen atoms than the corresponding alkane
What is the IUPAC name for a four-carbon continuous-chain alkane?
Butane
3 multiple choice options
According to the IUPAC convention for chemical naming, which part of the hydrocarbon is selected as the main chain for a hydrocarbon chain...
the longest continuous chain, regardless of bends
According to the IUPAC convention, alkyl group names should be located _______ of the name of the main chain
in front
According to the IUPAC convention, alkyl substituents on a hydrocarbon chain should be listed in which order?
alphabetical without considering prefixes
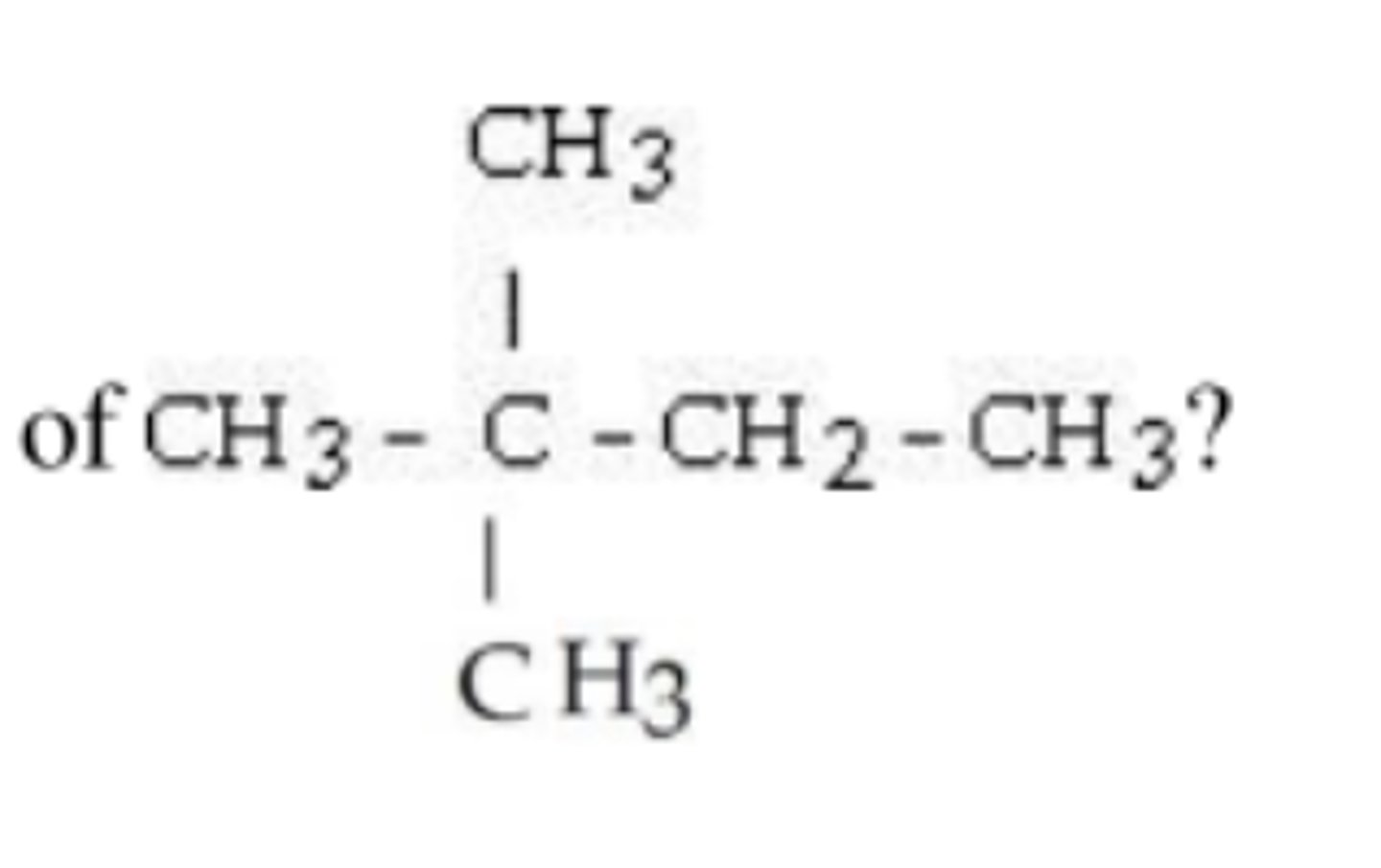
What is the IUPAC name of the following compound?
2-methylhexane
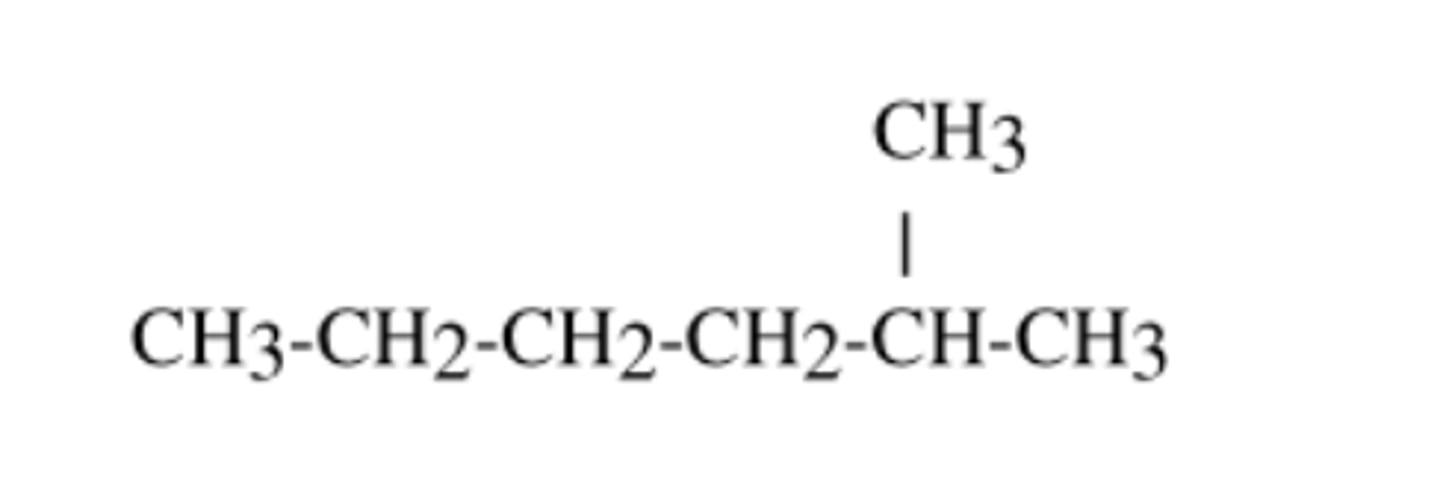
Compounds that have the same molecular formula but different arrangements of atoms are called _____
structural isomers
What is the IUPAC name of this compound?
1,3-dichlorobutane
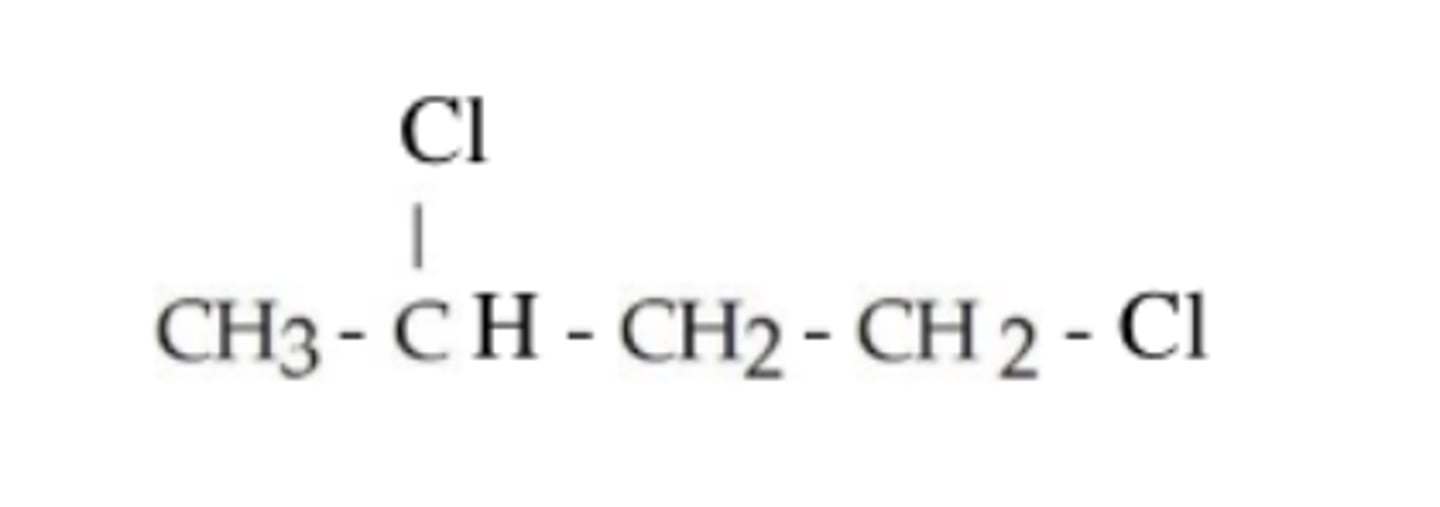
What is the IUPAC name of this compound?
2,4-dimethylpentane
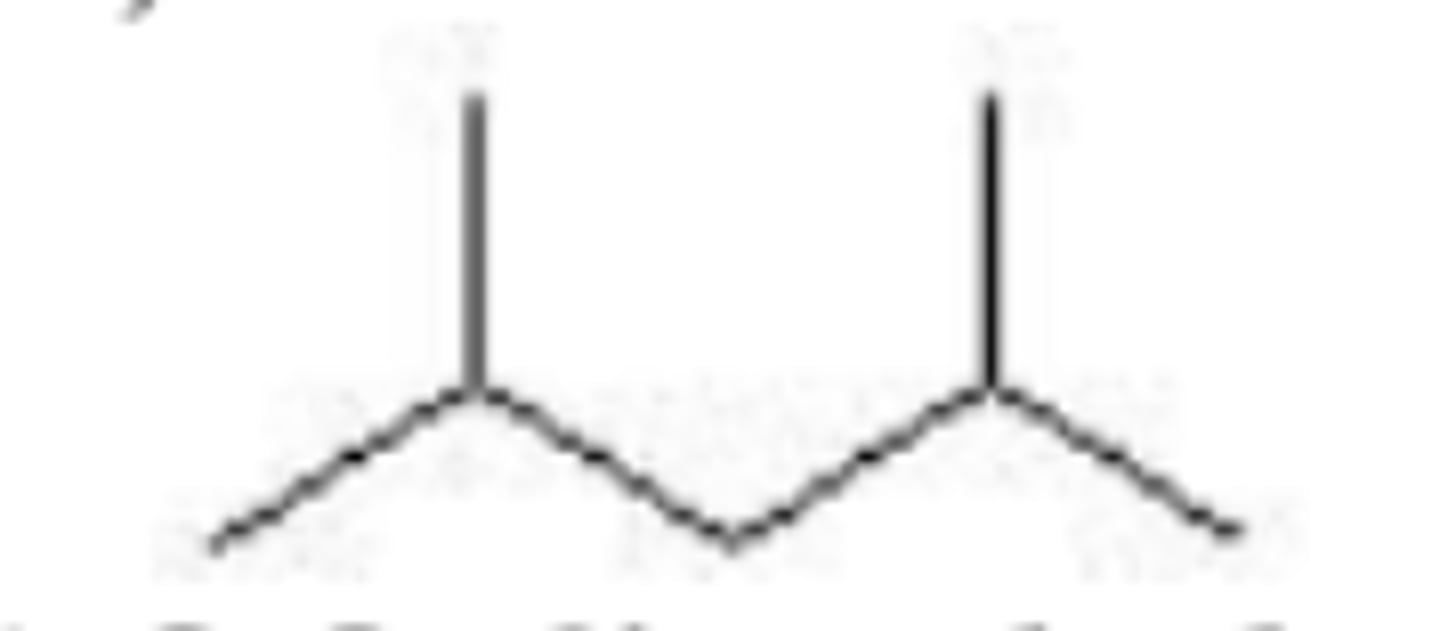
What is the IUPAC name of the alkyl group CH3-CH2-CH2?
Propyl
What is the IUPAC name for this alkane?
3,4-dimethylhexane
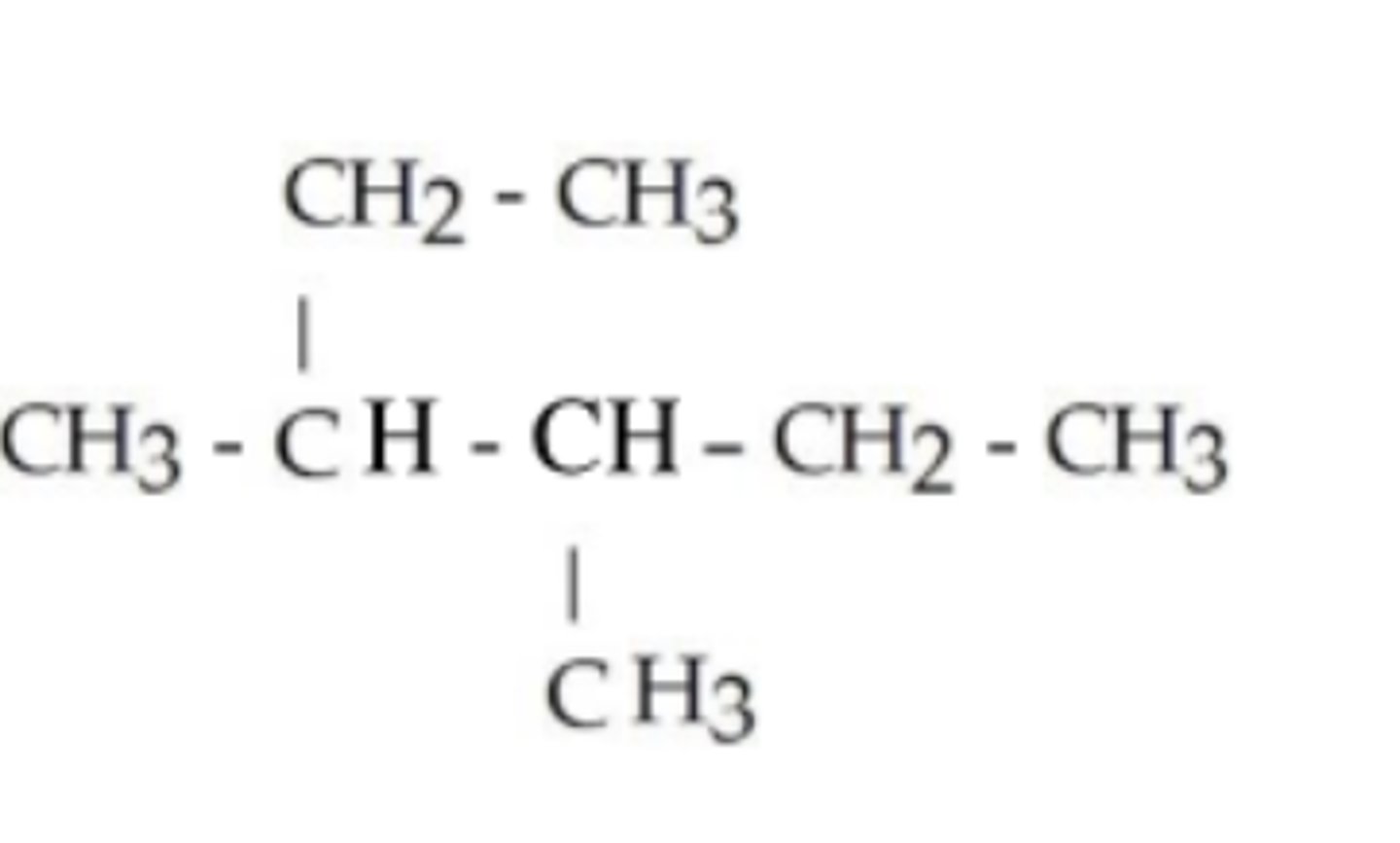
what is the IUPAC name of this alkane?
2,2,4-trimethylhexane
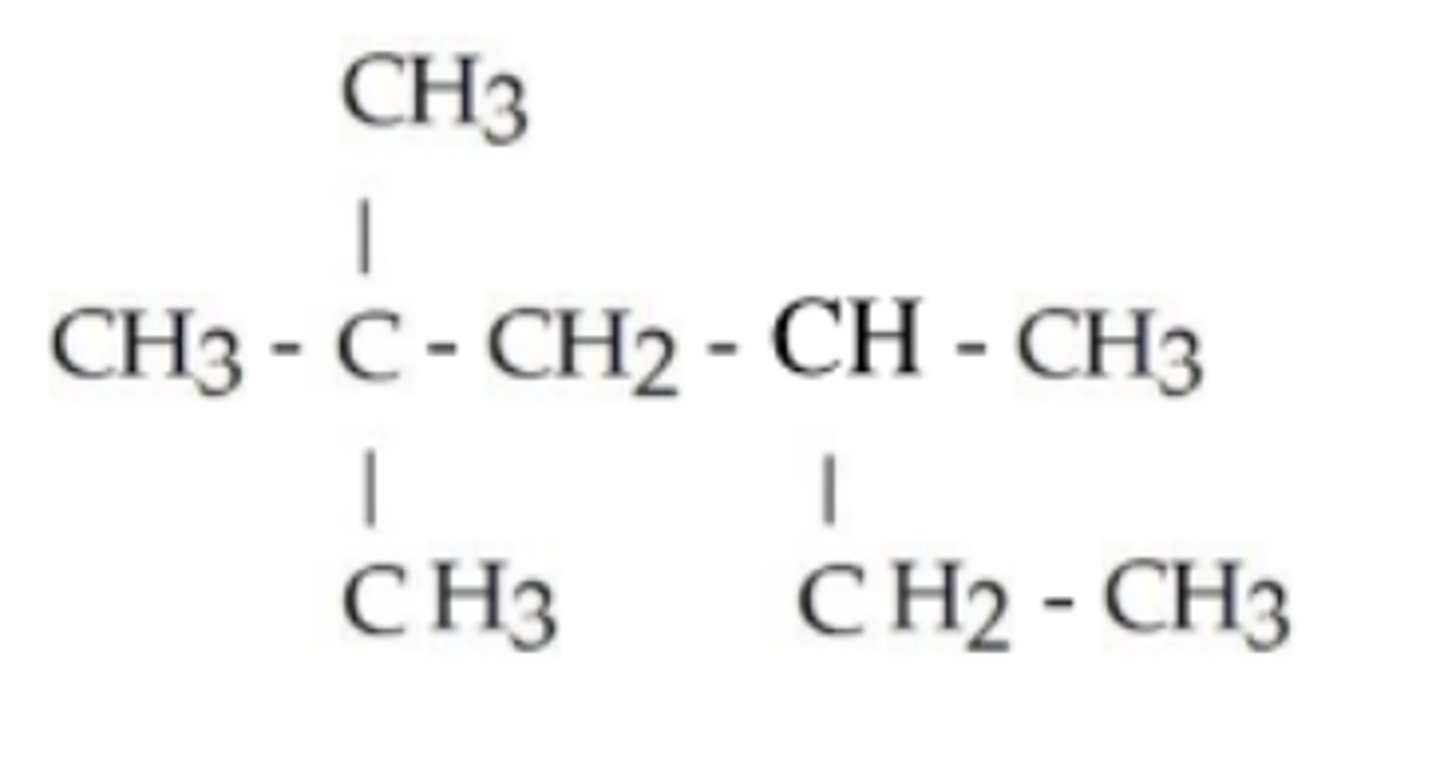
What is the IUPAC name of this compound?
2-methylpentane
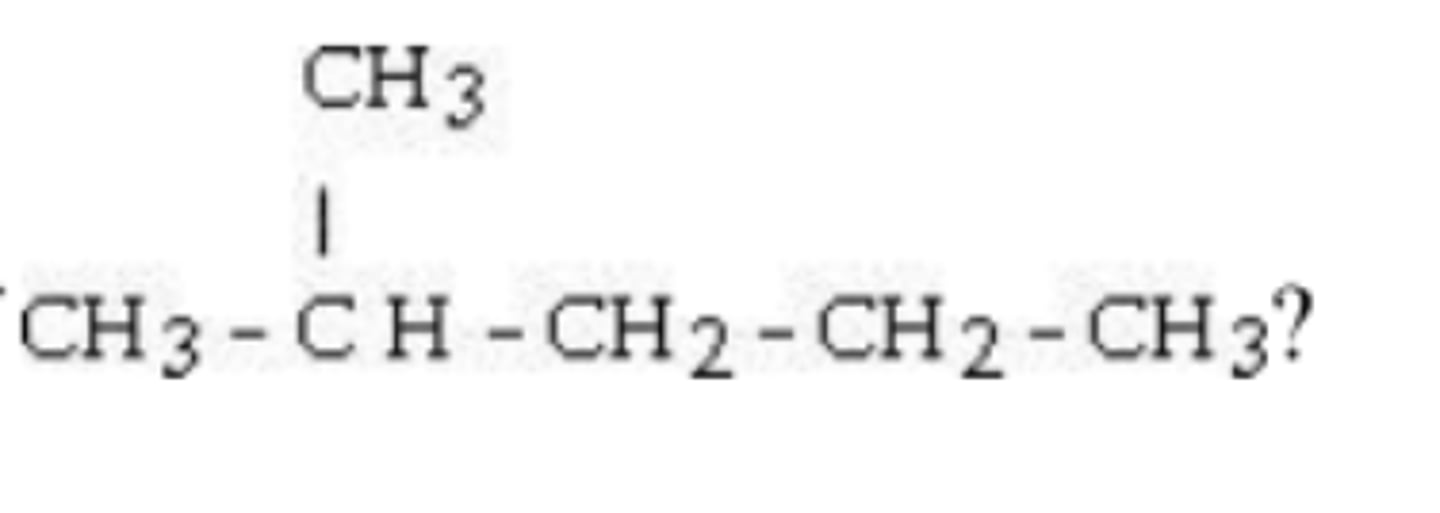
What is the IUPAC name of this compound?
2,2-dimethylbutane
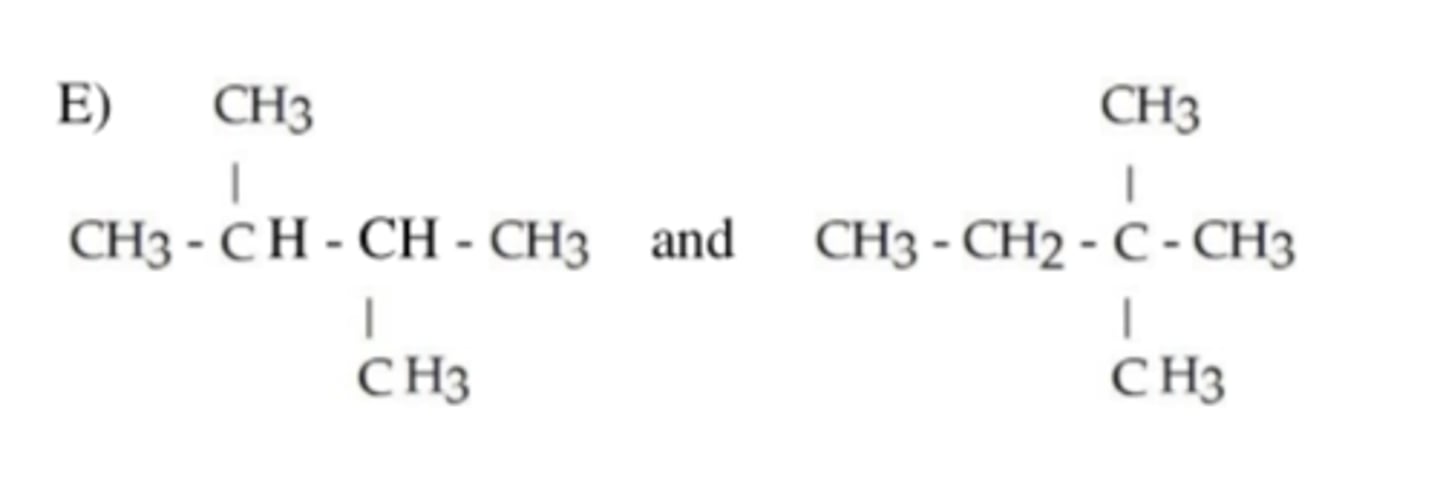
The following pairs represent an _____
isomer
The reaction of butane with oxygen is called _____
combustion
An alkene is an organic compound that contains a _____ bond
double
An alkyne is an organic compound that contains a ______ bond
triple
Alkenes and alkynes are called unsaturated compounds because _____
They have fewer hydrogen atoms attached to the carbon chain than alkanes
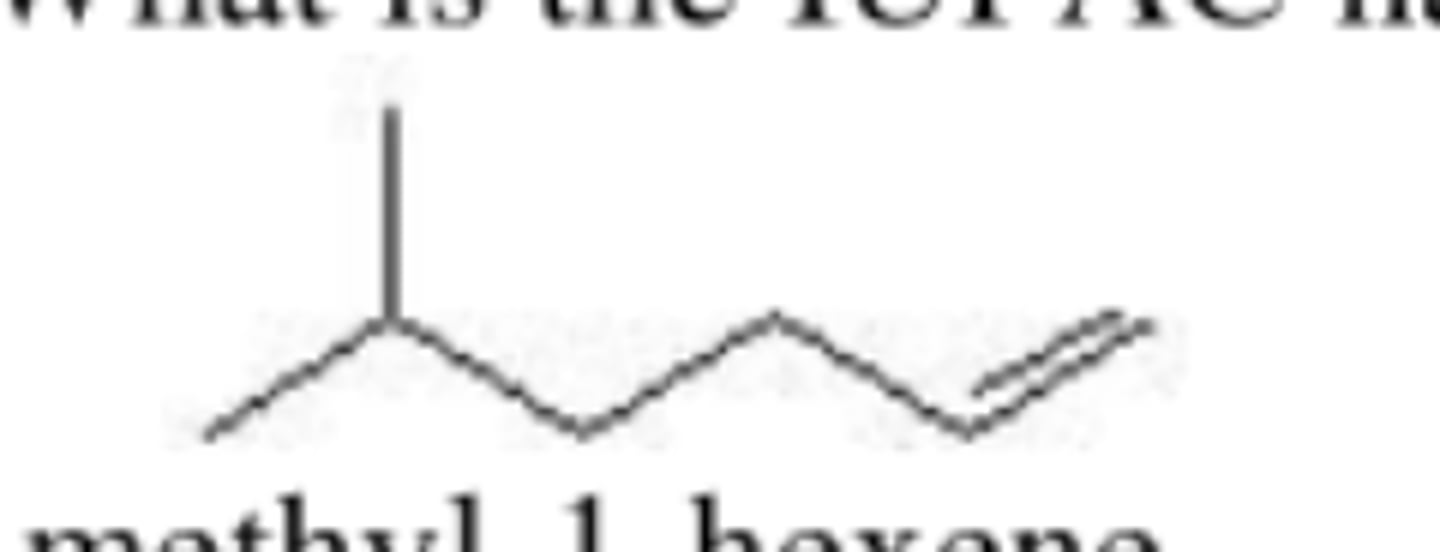
What is the IUPAC name of the following compound?
5-methyl-1-hexene
Organic compounds with carbon-carbon double or triple bonds are classified as ______
unsaturated compounds
The IUPAC name of CH3-CH=CH-CH3 is
2-butene
When naming an alkene, the parent chain is the longest carbon chain _____
that contains both atoms of the double bond
The IUPAC name for this compound is...
2-pentyne
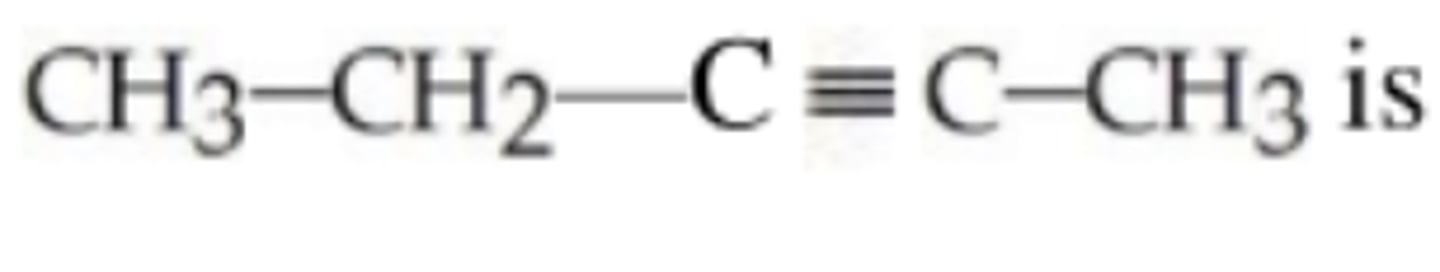
What is the IUPAC name for the following compound?
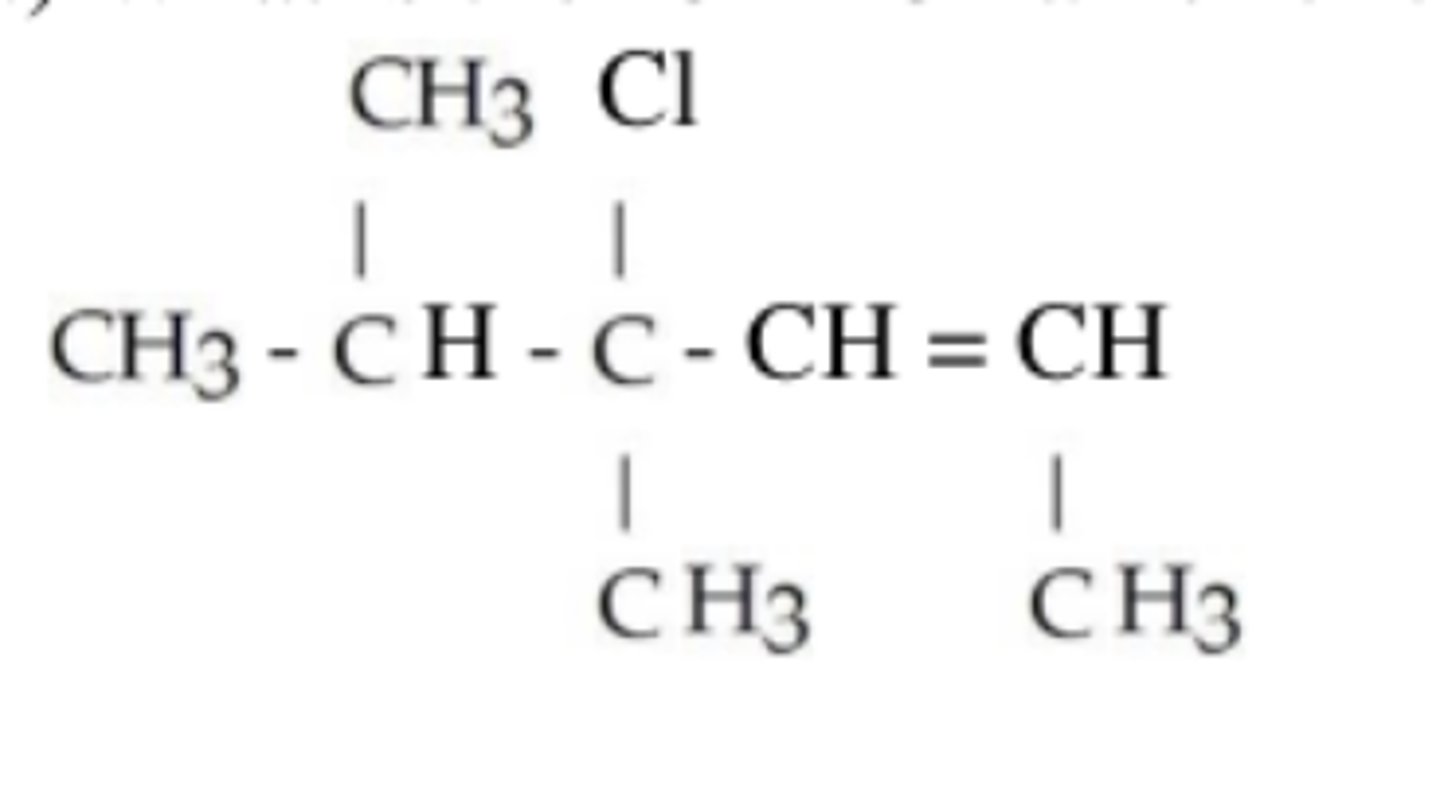
4-chloro-4,5-dimenthyl-2-hexene
What is the condensed structural formula of the compound propene?
CH3-CH=CH2
Some alkenes have geometric (cis-trans) isomers because _____
the carbon atoms in the double bond cannot rotate
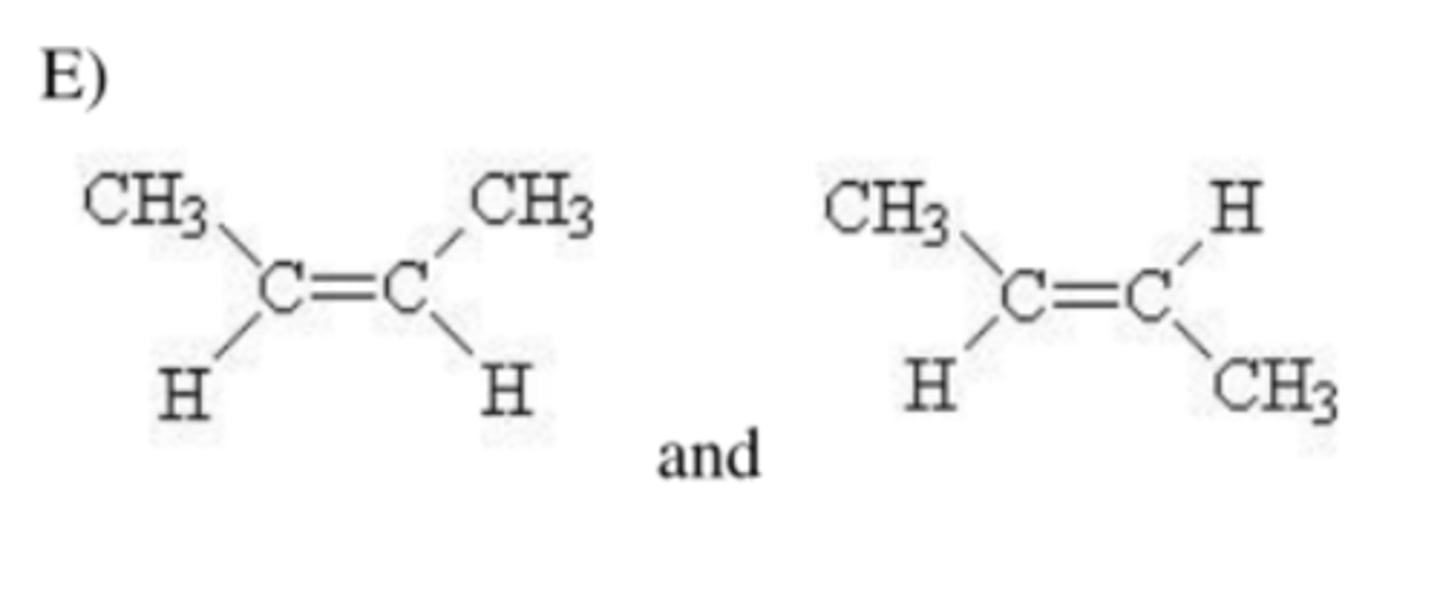
The pairs of compounds are _____
cis-trans isomers
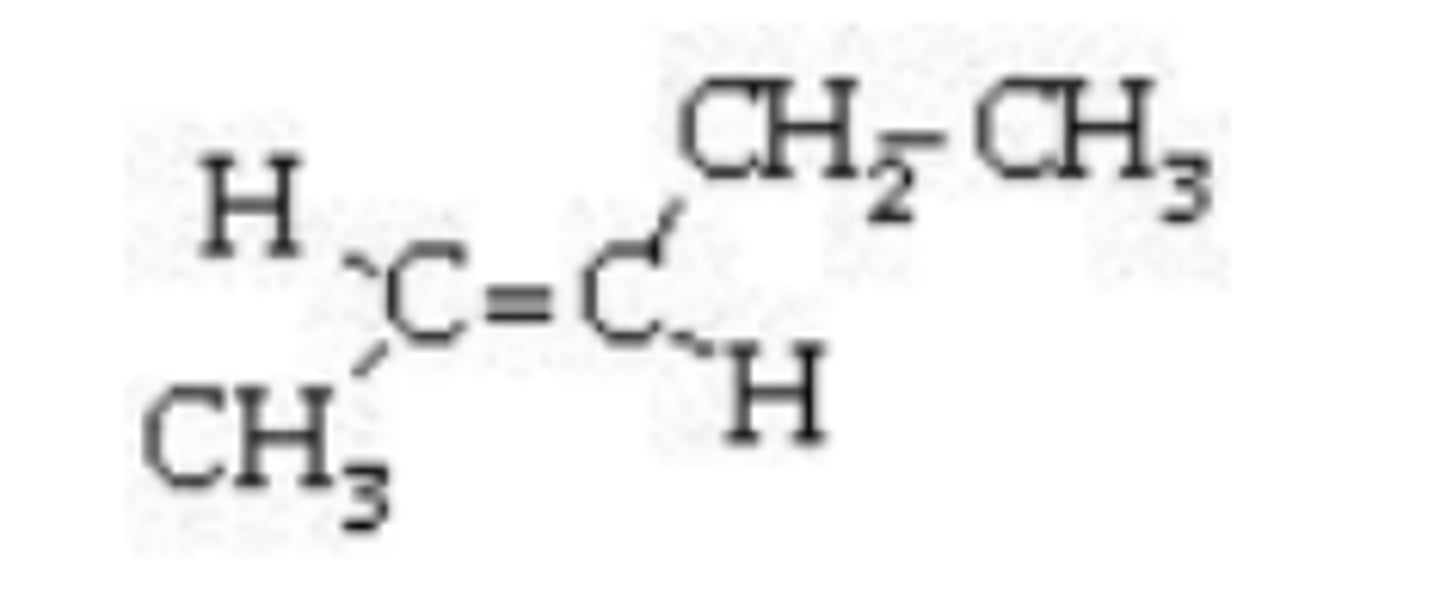
What is the IUPAC name of the compound shown?
cis-3-pentene
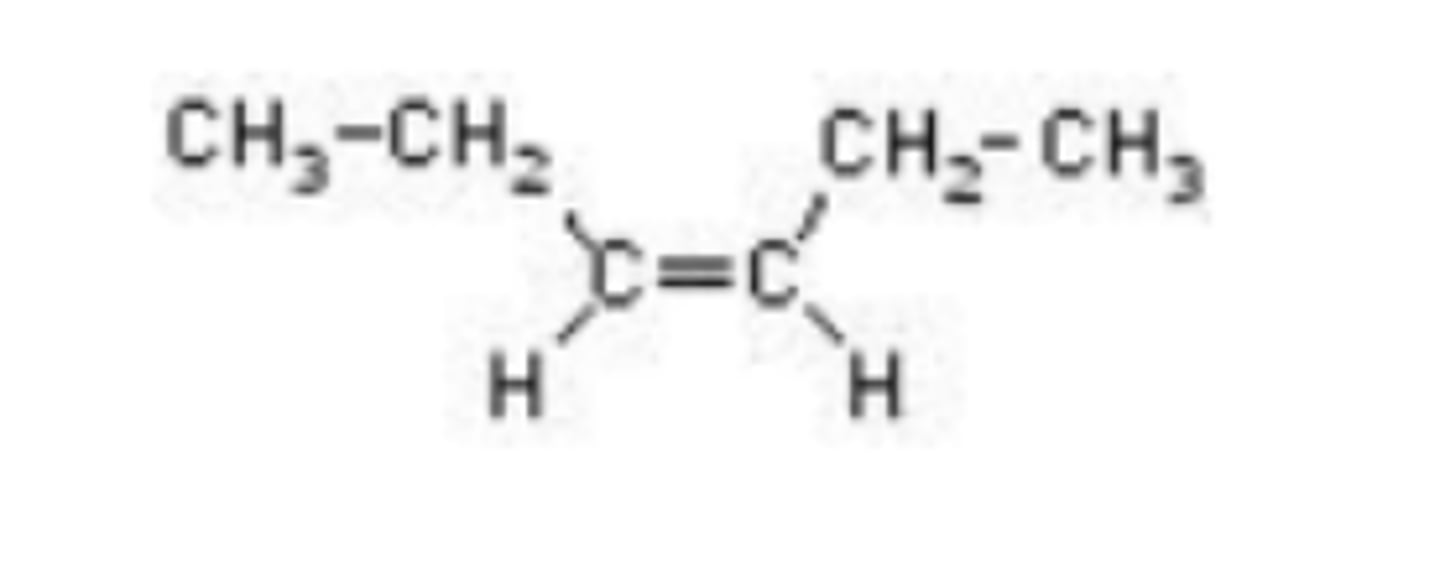
What is the IUPAC name of the compound shown below?
cis-3-hexene
The reaction of an alkene and water in the presence of an acid catalyst to produce an alcohol is called ______
hydration
What is the product of this reaction?
OH
CH3-CH2-CH-CH2
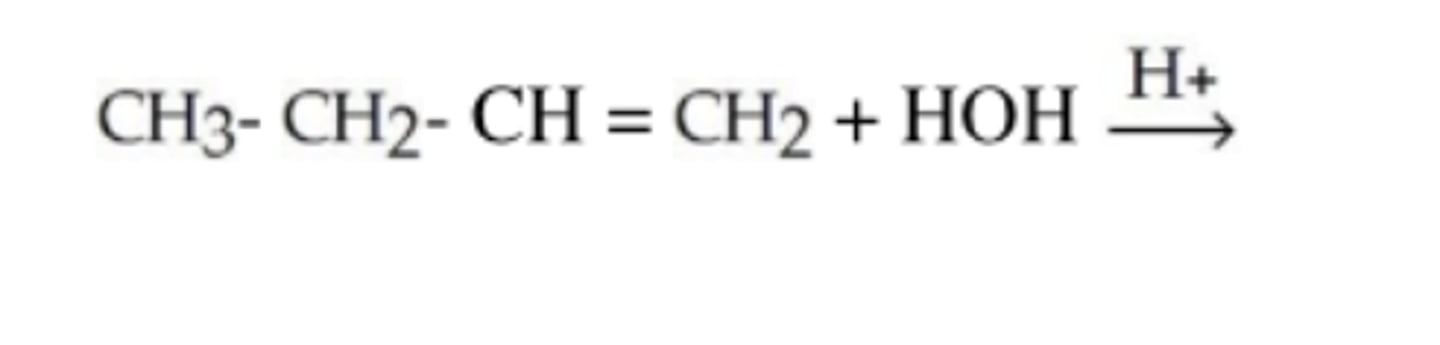
The compound is named _____
benzene
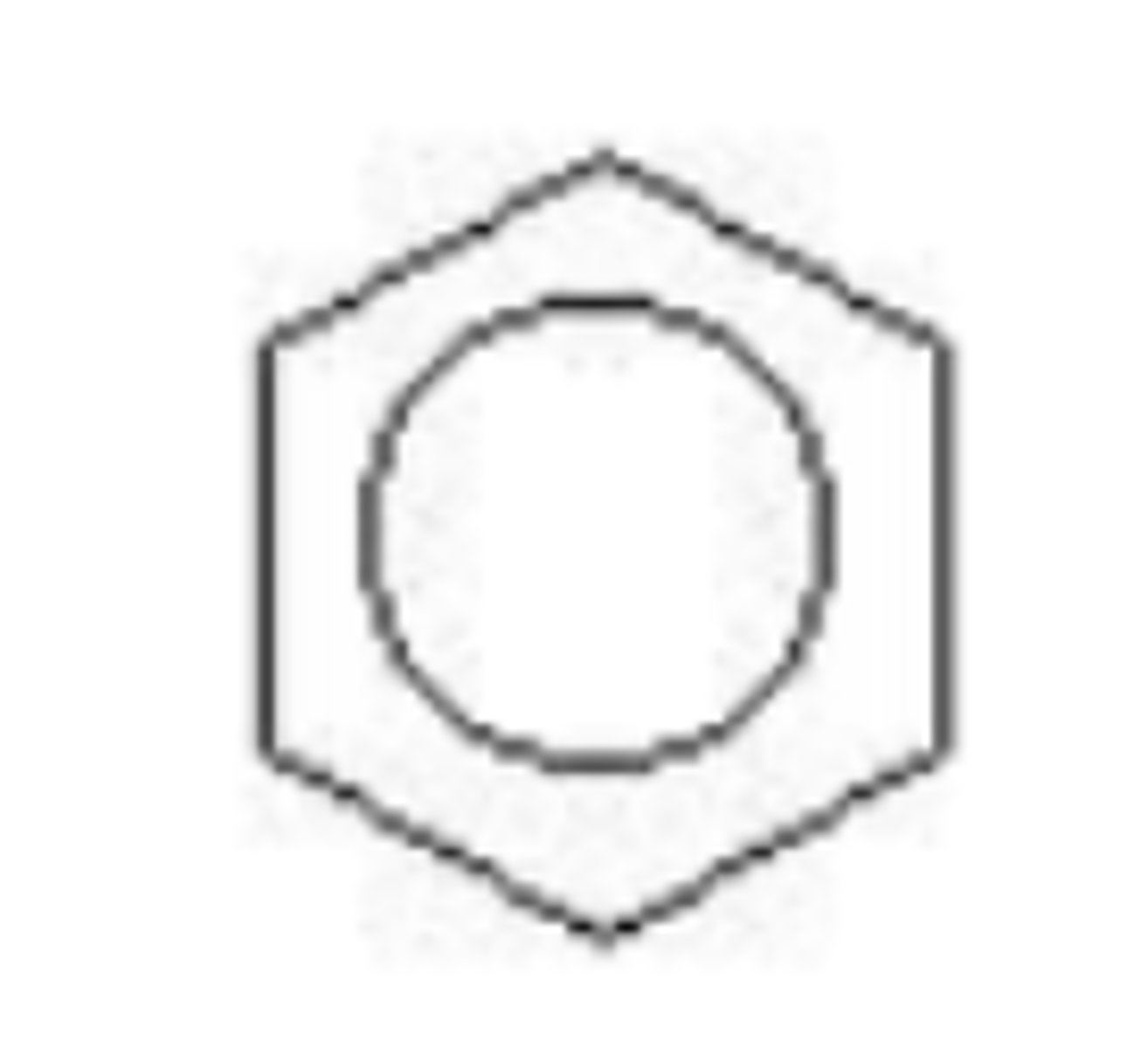
The compound below can best be described as _____
aromatic