VSEPR Shapes and Formulas
1/16
Earn XP
Description and Tags
27/5/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
e =
number of lone pairs
x =
number of bonding pairs
x = 2
AX2
linear
symmetrical
orbital hybridization: sp

x = 3
AX3
trigonal planar
symmetrical
orbital hybridization: sp2
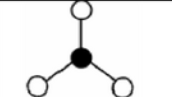
x = 2 e = 1
AX2E
trigonal planar: bent of v-shaped
non-symmetrical
sp2
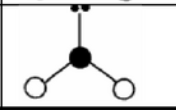
X = 4
AX4
tetrahedral
symmetrical
sp3
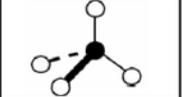
X = 3 e = 1
AX3E
tetrahedral: trigonal pyramidal
non-symmetrical
sp3
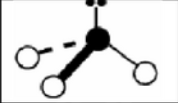
x = 2 e = 2
AX2E2
tetrahedral: bent or v shaped
non-symmetrical
sp3
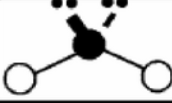
x = 5
AX5
trigonal bipyramidal
sp3d
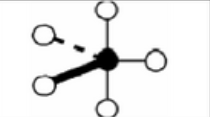
x = 4 e = 1
AX4E
trigonal bipyramidal: seesaw
non symmetrical
sp3d
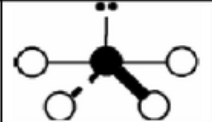
x = 3 e = 2
AX3E2
trigonal bipyramidal: t-shaped
sp3d
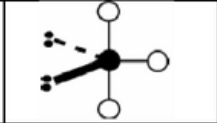
x = 2 e = 3
AX2E3
trigonal bipyramidal: linear
symmetrical
sp3d
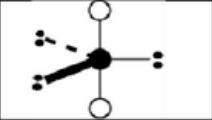
x = 6
AX6
octahedral
sp3d2
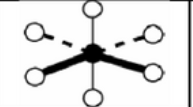
X = 5 E =1
AX5E
octahedral: square pyramidal
sp3d2
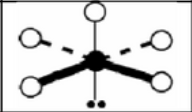
X = 4 e = 2
AX4E2
octahedral: square planar
sp3d2
non-symmetrical
bent
trigonal pyramidal
see saw
symmetrical shapes
linear
trigonal planar
tetrahedral