Synthesis of Benzocaine
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
2 stages
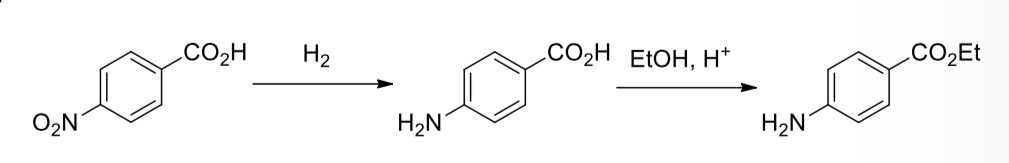
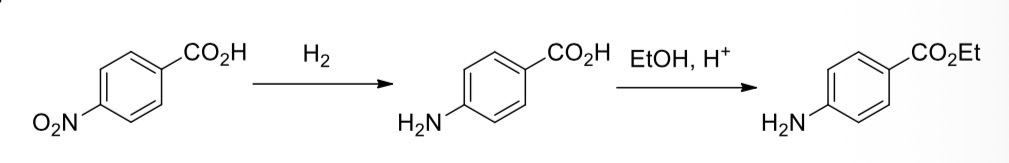
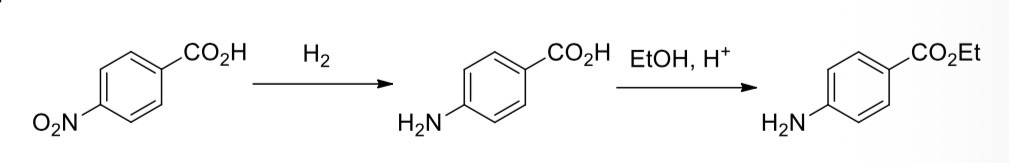
4-nitrobenzoic acid —> 4-aminobenzoic acid
4-aminobenzoic acid —> ethyl 4-aminobenzoate (benzocaine)
Part 1
Explain the reaction that occurs
4-nitrobenzoic acid —> 4-aminobenzoic acid
Reduction of the nitro group in 4-nitrobenzoic acid
Using tin metal in the presence of hydrochloric acid
Part 1
Method
4-Nitrobenzoic acid is reduced by refluxing with granulated tin and concentrated hydrochloric acid until a clear solution forms
After cooling, the solution is separated from residual tin and the tin is washed with water
The combined solution is treated with concentrated ammonia to make it basic, causing inorganic precipitates to form
The warm mixture is filtered to remove these precipitates. The filtrate is then acidified with glacial ethanoic acid to precipitate the product
Product is cooled, collected by vacuum filtration, and dried in an oven
Part 1
5g of 4-nitrobenzoic acid is used.
What is the theoretical yield of this reaction
1 mol 4-nitrobenzoic acid → 1 mol 4-aminobenzoic acid
Tin and HCl are in excess, so 4-nitrobenzoic acid is the limiting reagent
4-Nitrobenzoic acid (C₇H₅NO₄): 167.12 g mol⁻¹
4-Aminobenzoic acid (C₇H₇NO₂): 137.14 g mol⁻¹
moles of 4-nitrobenzoic acid= 5.00 g / 167.12 g = 0.0299 mol
theoretical yield = 0.0299 mol × 137.14 g mol = 4.10 g
Part 1
What is the inorganic precipitate formed after concentrated ammonia is added?
Tin hydroxide
Part 1
Why was concentrated ammonia solution used for the first neutralisation and not NaOH?
Ammonia is a weak base = so the pH rises more gradually = prevents sudden precipitation or decomposition of the organic product
Using NaOH (a strong base) would make the solution strongly alkaline, which could:
Convert 4-aminobenzoic acid into its water-soluble sodium salt, reducing product yield
Cause excessive or rapid precipitation of tin hydroxides that can trap or adsorb the product
Part 1
Why was ethanoic acid used for the final acidification instead of, for example, HCl?
Ethanoic acid is a weak acid = so the pH decreases gradually = allows the 4-aminobenzoic acid to precipitate slowly
Using HCl (a strong acid) would:
Protonate the amine to form the highly water-soluble ammonium chloride salt, keeping the product dissolved and reducing yield
Ethanoic acid avoids introducing high concentrations of chloride ions, which could keep residual tin species in solution or contaminate the product
Part 1
4-Aminobenzoic acid has two pKa values of 2.4 and 4.9. Assign these pKa values to the appropriate functional groups and explain your answer
4-Aminobenzoic acid contains two ionisable functional groups:
a carboxylic acid (–COOH)
an amine (–NH₂)
Assignment of pKₐ values
pKₐ ≈ 2.4 → carboxylic acid (–COOH)
pKₐ ≈ 4.9 → conjugate acid of the amine (–NH₃⁺)
Part 1
What is the biological relevance of 4-aminobenzoic acid (PABA)
Used for folate synthesis in microorganisms
Sulfonamide antibiotics act as structure analogues of PABA = blocks folate synthesis in bacteria = inhibits bacteria growth
Part 1
How could 4-nitrobenzoic acid be prepared starting from toluene?
Electrophilic nitration of toluene (para-selective)
Toluene is nitrated using a mixed acid (concentrated HNO₃ / concentrated H₂SO₄).
The methyl group is activating and ortho/para-directing, so nitration gives mainly:
o-nitrotoluene
p-nitrotoluene (major)
The para isomer can be isolated by fractional crystallisation
Oxidation of the methyl group to –COOH
The methyl side chain is oxidised to a carboxylic acid using a strong oxidising agent such as: Hot alkaline KMnO₄, followed by acidification
Forms 4-nitrobenzoic acid
Part 2
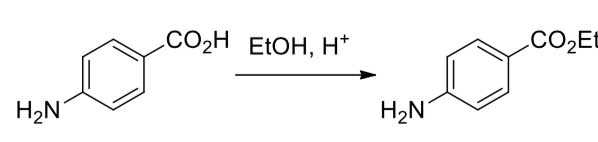
Explain the reaction that occurs
4-aminobenzoic acid —> ethyl 4-aminobenzoate (benzocaine)
Acid-catalysed (Fischer) esterication of the carboxyl group using ethanol
Part 2
Method
4-Aminobenzoic acid is dissolved in ethanol and carefully treated with concentrated sulfuric acid
It is heated under reflux to carry out esterification
After cooling, the reaction mixture is poured onto ice and neutralised with concentrated ammonia to make it alkaline, causing the ester product to crystallise
The solid product is collected by vacuum filtration, washed with cold water, and dried in a warm oven
Part 2
Why was concentrated sulphuric acid used for the reaction rather than, for example, concentrated hydrochloric acid? (Hint: consider water content)
The reaction is a Fischer esterification, which is an equilibrium process between a carboxylic acid and an alcohol
Concentrated sulfuric acid (~98%) has very low water content and can remove water formed during the reaction, shifting the equilibrium toward ester formation
Concentrated HCl is aqueous (typically ~37% HCl in water), introducing significant water into the reaction mixture, which would drive the equilibrium backward and reduce ester yield
Part 2
Explain why the product precipitated on addition of ammonia solution in Step 6. Include the term ‘pKa’ in your answer.
On addition of ammonia solution, the reaction mixture becomes alkaline
The ester product (from 4-aminobenzoic acid) contains an ammonium group which conjugate acid has a pKₐ of around 4–5
When the pH is raised above this pKₐ, the protonated amine (–NH4⁺) is deprotonated to the neutral –NH₂ form.
The neutral molecule is much less soluble in water = precipitates out of solution
Part 2
What are the typical pharmaceutical applications of benzocaine?
Local anaesthetic
Part 2
Benzocaine mechanism of action
Blocks voltage-gated sodium channels, preventing nerve impulse conduction
Part 2
What potential stability problems might there be with formulating benzocaine as an injection?
How might the pH of the injection influence this?
1. Poor aqueous solubility
Benzocaine is largely unionised at physiological pH because it is a weak base (an amine) = poorly water soluble
In an injection, this would lead to precipitation
2. pH effects on ionisation
To increase solubility, the formulation would need to be acidic (pH < pKₐ) so the amine is protonated
Very acidic pH would be irritating and painful on injection.
On injection into the bloodstream (pH ~7.4), the drug would rapidly deprotonate and precipitate in situ
3. Chemical instability (ester hydrolysis)
Benzocaine is an ester, which is susceptible to hydrolysis:
Acid-catalysed hydrolysis at low pH
Base-catalysed hydrolysis at high pH
Maintaining a pH that improves solubility could therefore accelerate degradation, reducing shelf life
Part 2
Is the amino group in benzocaine a stronger or a weaker base than an aliphatic amine like ethylamine? Explain your answer.
The amino group in benzocaine is a weaker base than an aliphatic amine such as ethylamine:
Benzocaine
The amino group is an aniline-type amine attached to an aromatic ring
The nitrogen lone pair is delocalised into the benzene ring by resonance = less available to accept a proton
Additionally, the electron-withdrawing ester group on the benzene ring further reduces the basicity of the amino group by induction
Ethylamine
Aliphatic amine
Its lone pair is localized on nitrogen and is further stabilised by the electron-donating alkyl group
.
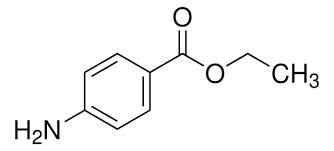
Part 2
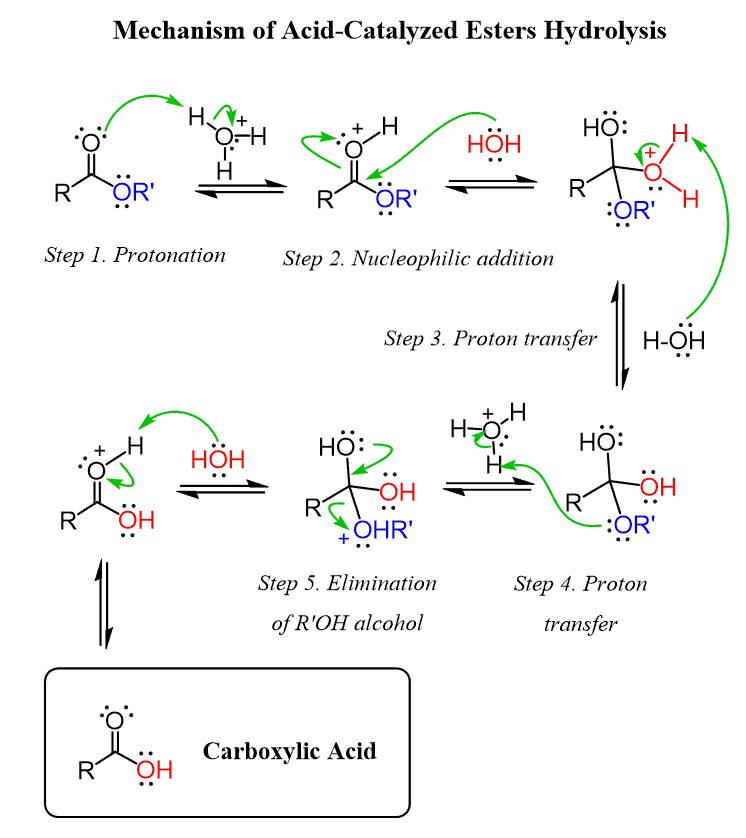
Write down a mechanism for the hydrolysis of the ester group in benzocaine.
Would you expect the amino group to exert an electronic influence to speed up or slow down the reaction?
The amino group slows down ester hydrolysis:
The –NH₂ group is electron-donating by resonance
It donates electron density into the aromatic ring and toward the ester carbonyl, making the carbonyl carbon less electrophilic
A less electrophilic carbonyl is less susceptible to nucleophilic attack
Part 2
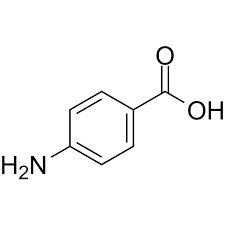
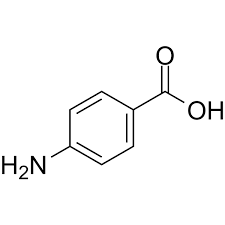
What is the molecular formula of 4-aminobenzoic acid
C7H7NO2
Part 2
What is the molecular weight of 4-aminobenzoic acid to the nearest whole number?
137

Part 2
What is the molecular formula of ethyl 4-aminobenzoate (benzocaine)?
C9H11NO2
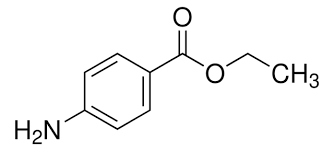
Part 2
What is the molecular weight of ethyl 4-aminobenzoate (benzocaine) to the nearest whole number?
165