Halogenoalkanes
1/46
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
47 Terms
What are Halgoenoalkanes?
Halogenoalkanes are also known as haloalkanes or alkyl halides. Simply put, they are alkanes, but they contain a halogen atom instead of one (or more!) of their hydrogen atoms. The halogen atom is referred to as X. The general formula is CnH2n+1
Reactivity of halogenoalkanes compared to alkanes
Halogenoalkanes are generally more reactive than alkanes due to the presence of a halogen atom, which is more electronegative than carbon. This results in a polar C-X bond (where X is the halogen), making it susceptible to nucleophilic attack.
What is nomenculture (halogenoalkanes)
Straight chain halogenoalkanes are named according to the parent alkane. The longest continuous straight chain of carbon atom is selected and numbered in order to indicate the position of the halogen substituent on the chain. The end of the chain starts is chosen so as to use the lowest values. The name of the halogen prefixes the name of the parent alkane.
What are the classes of halogenoalkanes
They can be classified as primary (1o) secondary(2o) tertiary (3o) according to their structure
How does an alkane reaction with chlorine to form a halogenoalkane
Alkanes react with chlorine explosively in the presence of ultra violet light
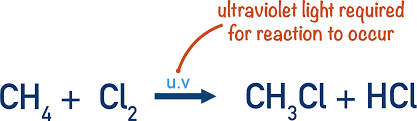
Why would there no reaction if a reaction to make a halogenoalkane was not done use UV light
The reaction in the presence of UV light is very fast as it takes place via a free radical substitution mechanism. The reaction has a number of distinctive steps:
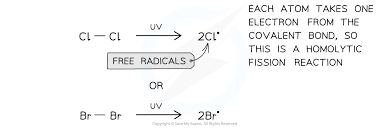
What is a free radical?
A species with an unpaired electron
What are the 3 steps involve in a reaction between an alkane and halogen
Initiation step. propagation step and termination step
What is the initiation step (Chlorine and methane)
This step involves the breaking of the Cl-Cl covalent bond forming 2 Cl atoms (or radicals) by exposing the chlorine molecule to UV light. The Cl-Cl bond breaks as it is the weakest bond.
Propagation step (Chlorine and methane)
This step involves the chlorine atom attacking a methane molecule forming a methyl radical. The methyl radical further reacts with another chlorine molecule forming chloromethane and another Cl atom. Hence the chain is propagated
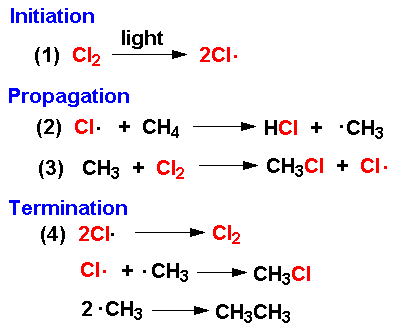
Termination step (Chlorine and methane)
The reaction is terminated whenever radicals are removed from the reaction, it is very easy for such a fast reaction as this to quickly build up a mixture of products. The chloromethane is also reactive towards chlorine radicals and is quickly converted into dichloromethane. In order to limit a further reaction, an excess of CH4 is used.
What is always formed during the first propagation step
A hydrogen halide
What are the uses of halogenoalkanes
They can be used as solvents as they tend to be unreactive liquids which are good at dissolving other polar organic compounds. The chlorofluoroalkanes are often called CFCs ( chloro-fluoro carbons) and widely used as the coolant in fridges.
Why is ozone good?
Ozone formed naturally in the upper atmosphere is beneficial as it absorbs harmful ultra-violet radiation which damages cells and can cause skin cancer.
How did halogenoalkanes build up in the atmopshere, what did it cause and why is this a problem?
The chlorofluoroalkanes are very unreactive and do not dissolve in water so they diffused gradually over many year up into the upper atmosphere. Here the ultra violet light decomposed the molecule forming chlorine atoms as the energy broke the C-Cl bonds. The chlorine atoms catalyse the decomposition of ozone and contributed to the formation of a hole in the ozone layer
How does electronegativity work in halogenoalkanes
The halogen atoms are much more electronegative than the C-atom (with exception of I) and, therefore, the pair of electrons forming the C-X bond is displaced towards the halogen. Hence, the bond is polarised and has some ionic character.
What influences the rate of reaction in a halogenoalkane
The strength of the carbon -halogen bond
Why are fluoroalkanes slow to react and how does the reactivity increase between the halogens?
Although the C-F bond is very polar it is very strong so fluoroalkanes are slow to react. Reactivity increases as follows:
CH3F<CH3CL<CH3Br<Ch3I
What are nucleophiles classed as?
They are classified as electron pair donors,; they are negatively charged ions or compounds in which an atom has a lone pair of electrons
How does nucleophilic substiution work?
Nucleophilic substitution occurs when a nucleophile, an electron pair donor, attacks a carbon atom in a halogenoalkane, which is bonded to a halogen (the leaving group). The nucleophile replaces the halogen in a two-step process
What are the 2 steps of nucleophilic substitution
Formation of a carbon-nucleophile bond: The nucleophile approaches the carbon, forming a temporary bond while the C-X bond begins to break.
Leaving group departure: The halogen, now with an extra pair of electrons, leaves as a halide ion, completing the substitution. This results in the formation of a new compound with the nucleophile attached to the carbon.
Why is an electron pair displaced towards the C atom in the C-X bond
The halogen atoms are much more electronegative than the C-atom (with the exception of I) and, therefore, the pair of electrons forming the C-X bond is displaced towards the halogen. Hence, the bond is polarised and has some ionic character.
What is the rate of reaction influenced by in halogenoalkanes
The strength of the carbon-halogen bond. As the halogen gets larger the bond strenght decreases, greater distance and shielding between nucleus and shared pair of electrons so weaker attraction
Reactivity pattern of halogenoalkanes
CH3F< CH3Cl<CH3Br<CH3I
What do nucleophilc reagants attack in terms of Halogenoalkanes
they attack the electron deficient end of the C-X bond. The halogenoalkanes are susceptible to he nucleophilic attack due to the Cs+.
What substance/compound is used during nucleophilic substitution normally
warm aqueous sodium hydroxide
Nucleophilic substitution image and explanation
The curly arrows represent the movement of electron pairs. The :OH- acts as a nucleophile attacking the slightly positive C-atom and donating its lone pair of electrons. The mechanism shows that a C-OH bond is formed and at the same time the C-Br bond breaks. The reaction results in the overall substituion of an OH group for a -Br atom. So it is a nucleophilic substituiton reaction
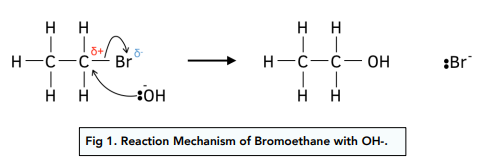
What does nucleophillic substitution involving sodium hydroxide always produce?
An alcohol
What raegents are used in the nucleophilic substitution between cyanide ions
KCN or NaCN
Nucleophilic substitution with cyanide image
fully dissociates to :CN ions
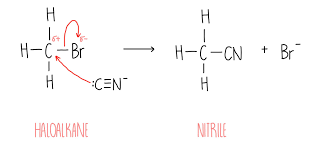
What do these reactions from cyanide always form?
A nitrile
Why is nucleophilic substitution with cyanide useful in industry
Carbon chain increases by one
Nucleophillic substituion with aqueous ammonia
CH3CH2Br + 2NH3 → CH3CH2NH2 =NH4Br
(bromoethane) +(excess ammonia) → (ethylamine) +(ammonium bromide)
Why is excess ammonia normally used with nucleophilic substitution
To minimises the chance of further reactions producing secondary and tertiary amines.
What is used to represent the movement of electron pairs
curly arrows
Nucleophillic substiution mechanism with ammonia
furthest to the right nitrogen has a lone pair of electrons
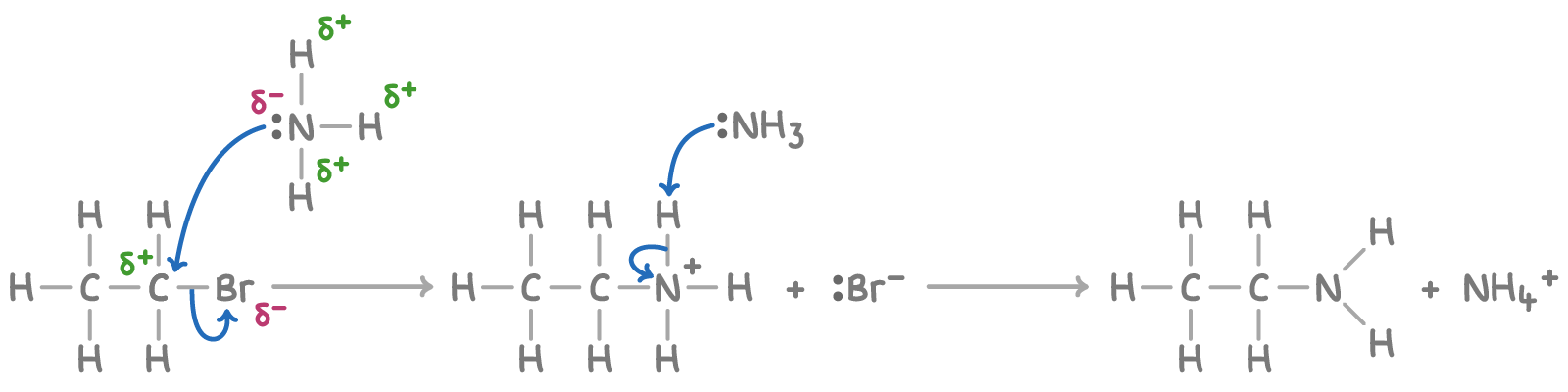
2nd NH3 attack explained in nucleophillic substitution
The NH3 molecule possesses a lone pair of electrons on the N atomn and hence, acts as a nucleophile. As the N is providing both electrons to form the new C-N bond, it acquires a positive charge. The HBr released reacts with NH3 to form NH4Br.
Why is nucleophillic substitution with NH3 a poor method to make amines industrially.
Further substitution ocurrs which leads to a mixture of products forming which have to be separated
What can we do to make amines efficiently
When using nucleophilic substitution use excess NH3 because it minimizes the chance of separation having to occur. Use a nitrile intermediate instead (beware it is toxic) you can slo use reduction/ hydrogenation
Mechanism for Elimination

Explanation of elimination mechanism
The lone pair on O forms coordinate bond with H this forces shared pair of electrons on C-H bond to form C=C. This repels electrons from C-Br bond onto Br and C-Br bond break
Which classes of halogenoalkanes are favoured but substitution or elimination
Primary → Substitution
Secondary → depends on conditions
Tertiary → depends on conditions
When can you only get elimination?
With a strong base acting as the nucleophile. (NaOH/ KOH) (Not NH3)
How can you make NaOH a stronger base?
By dissolving it
What temperatures favour substitiution/ elimination
Low temperatures favours substitution
High temperatures favour elimination
Overall what conditions are elimination reactions favoured by
Hot, ethanoic, KOH or NaOH and tertiary structures
Overall what are substitution reactions favoured by?
Warm aqueous, KOH or NaOH