Biogeochemical signalling
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
Nutrient recycling involves _______ and ________ reactions
oxidation & reduction
how can we summarise the C cycle?
The carbon cycle can be summarized as the process by which carbon atoms move through the Earth's systems, including the atmosphere, biosphere, oceans, and geosphere, through various processes such as photosynthesis, respiration, decomposition, and combustion.
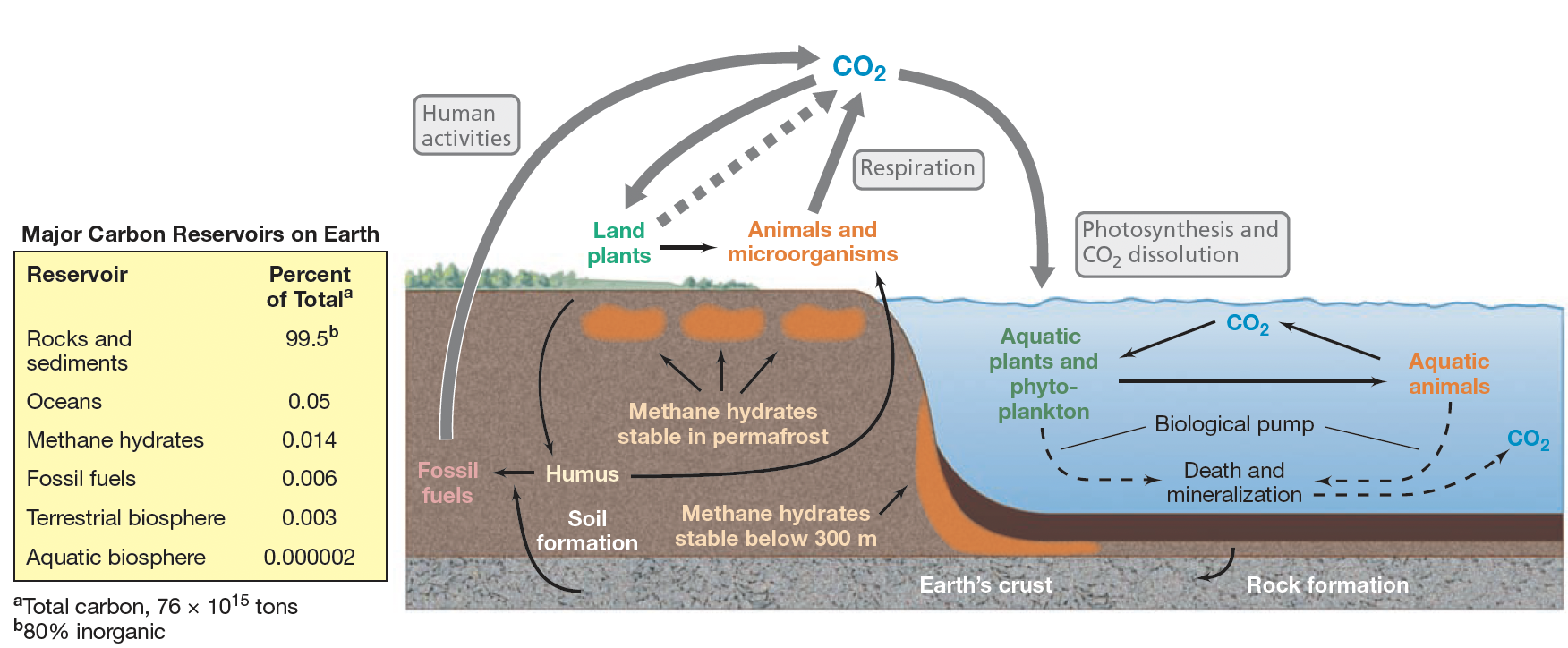
Are the C and O2 cycles interlinked?
Yes, C and O cycles are closely connected, as oxygenic photosynthesis both removes CO2 and produces O2, and respiration both produces CO2 and removes O2.
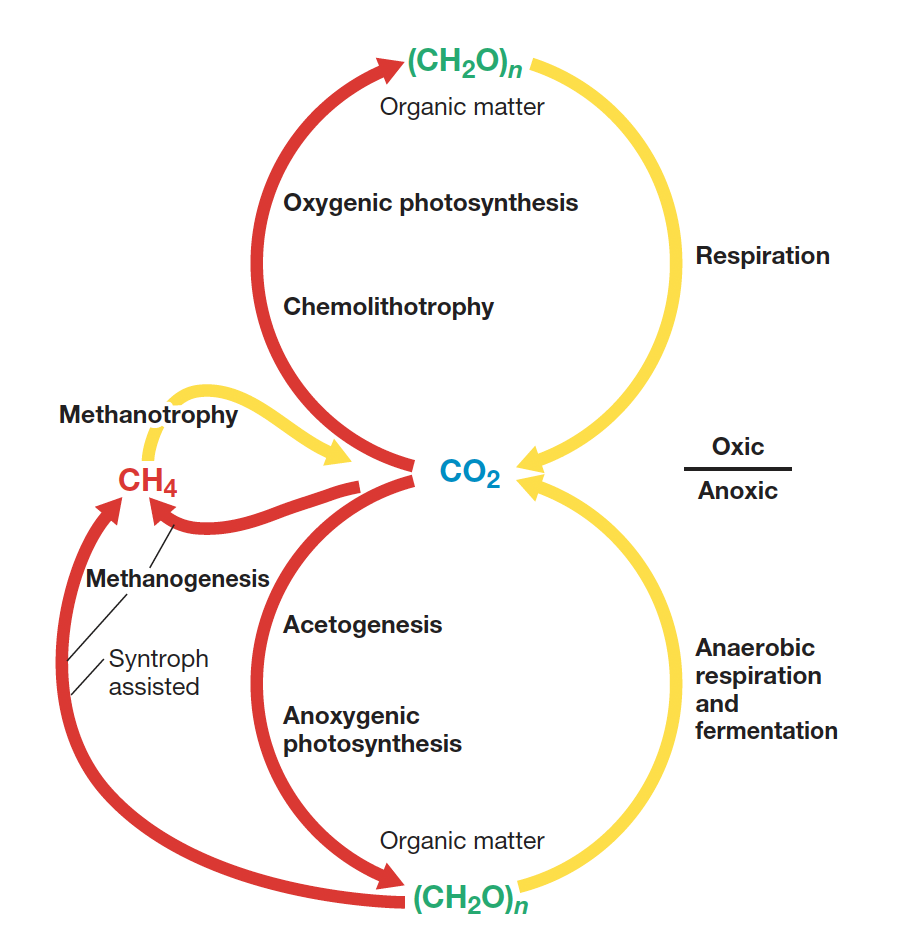
What is the Redox C cycle?
Describes the series of oxidation and reduction reactions involving C, where carbon compounds are oxidized to produce CO2 i.e by fermentation and reduced in processes like photosynthesis / methanogenesis
why is it important to understand how nutrient cycles are interconnected for microorganisms?
Understanding the interconnection of nutrient cycles is crucial for microorganisms as it influences their metabolic processes and ecological roles.
It helps in predicting how changes in one cycle can affect others, impacting nutrient availability and ecosystem health.
Eg: High levels of nitrate (NO3−), an excellent N source for aquatic phototrophs, stimulate primary production but also increase the rate of denitrification; the latter removes fixed forms of N from the environment and feeds back in a negative way on primary production
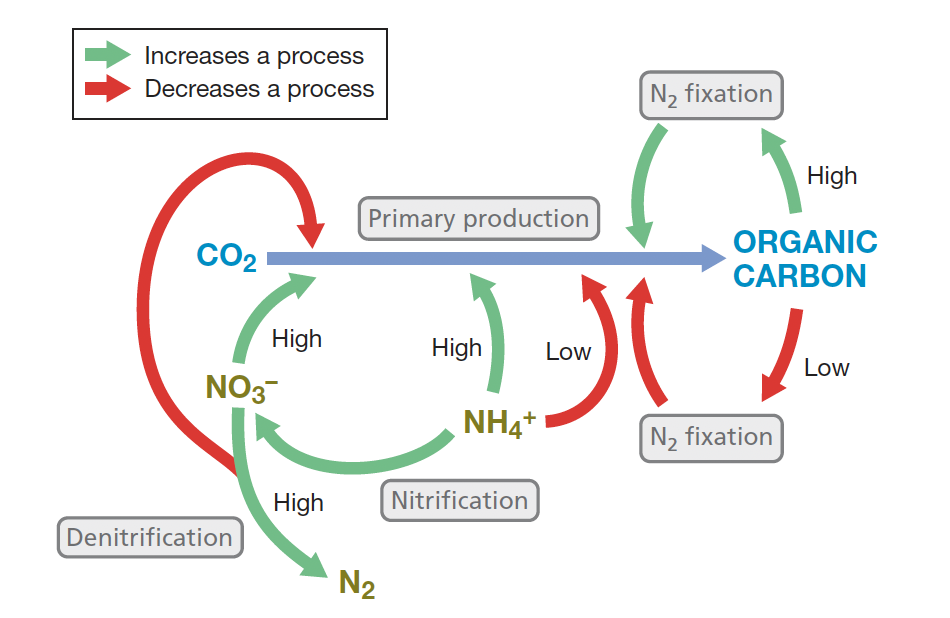
what is methanogenesis & which microbes are responsible?
Metabolic process that produces methane (CH4) primarily carried out by Archaea known as methanogens (strict anaerobes) predominant in anoxic environments such as wetlands and the digestive tracts of ruminants.
Most methanogens use CO2 as a terminal e- acceptor in anaerobic respiration, reducing it to CH4 with H2 as electron donor.
Few other substrates like acetate, are directly converted to CH4 by methanogens
How is S cycled through ecosystems?
The cycling of sulfur (S) involves processes such as anaerobic reduction, oxidation , desulfurylation, where sulfur is transformed through biological and geological activities.
Key players include microorganisms that convert sulfate to sulfide and vice versa, influencing soil fertility and ecosystem productivity.
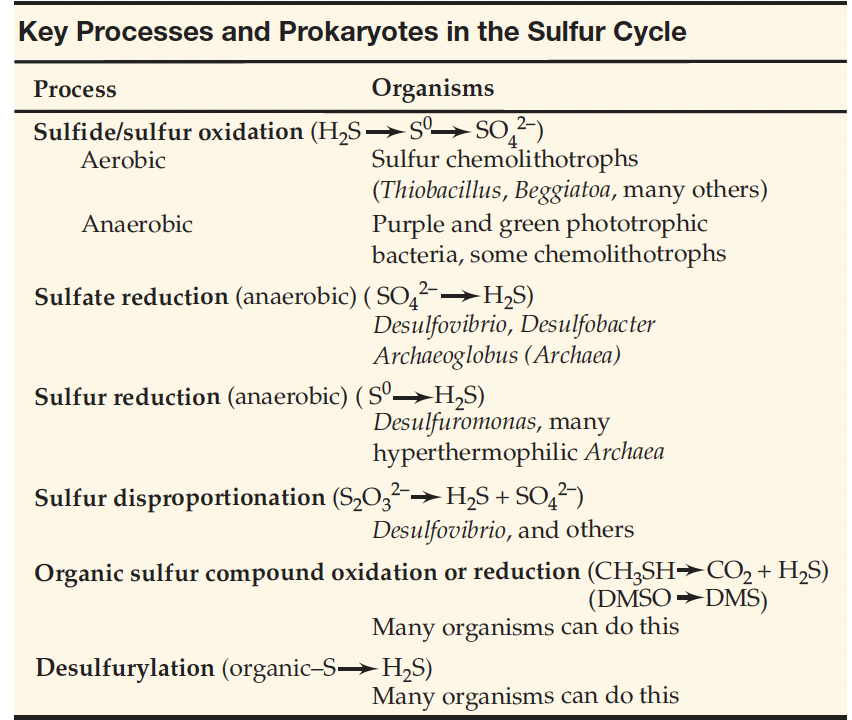
where is H2S produced?
Hydrogen sulfide (H2S) is produced from bacterial sulfate reduction (SO42– + 4 H2 → H2S + 2 H2O + 2 OH–) or is emitted from sulfide springs and volcanoes serving as an e- donor for chemolithotrophs to generate ATP
Is H2S a substrate or a product of the sulfate-reducing bacteria of the chemolithotrophic sulfur bacteria?
H2S is primarily considered a product of sulfate-reducing bacteria, formed through the reduction of sulfate (SO42–) during their metabolic processes, which facilitates energy production.
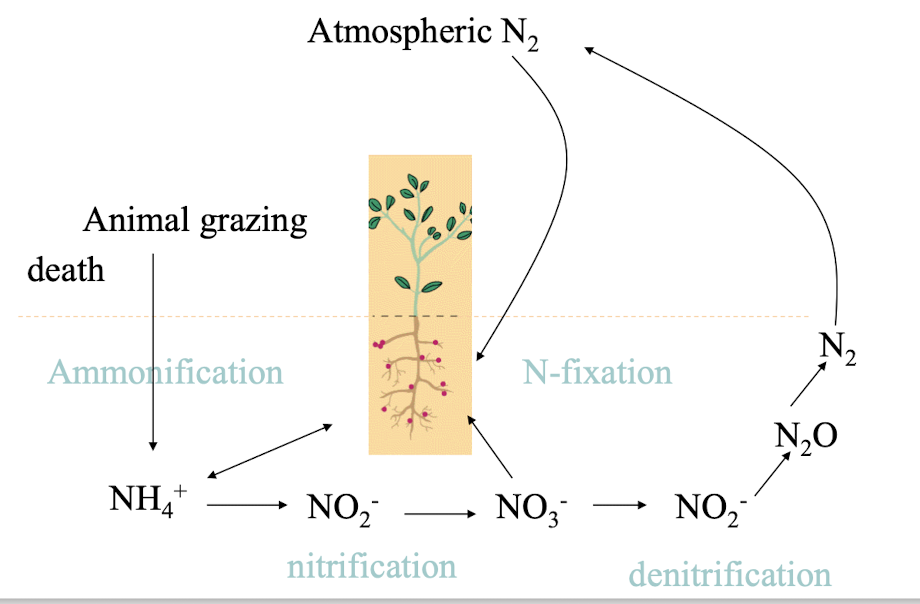
what are the 4 key features of the N cycle?
N fixation, where atmospheric N2 is converted to ammonia (NH3);
Nitrification, the process of oxidising ammonia to nitrate NH3 → NO3–
Denitrification, converting nitrate back to nitrogen gas (N2); and
Ammonification, where organic N is decomposed to ammonium (NH4+)
what is the chemical formula of N2 fixation & why is it very energy intensive if done manually?
Involves the reaction of atmospheric N2 with hydrogen (H2) to produce ammonia (NH3):
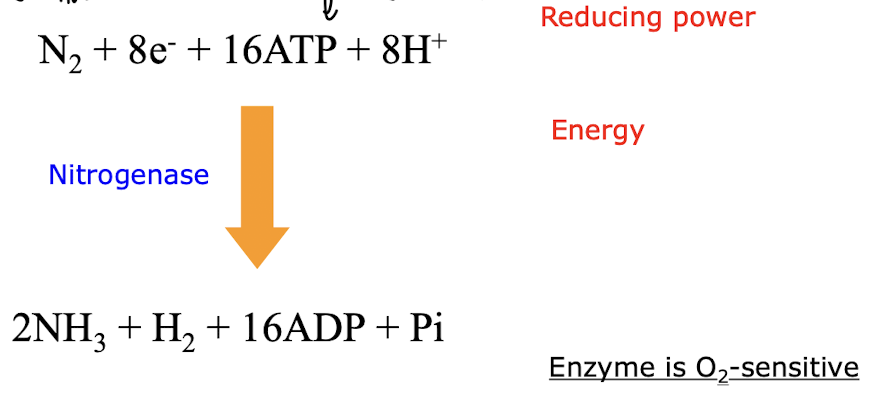
The process is energy-intensive manually due to the high temperatures and pressures required to break the triple bond in N2, necessitating significant energy input i.e using ATP
how is N2 naturally fixated by free living Azotobacter?
Azotobacter naturally fixes N2 by using the enzyme nitrogenase to convert atmospheric nitrogen into ammonia (NH3) under ambient conditions, facilitating nitrogen assimilation in the soil.
This process also helps enhance soil fertility by providing a natural source of N for plants for Rhizobium
How can Rhizobium fix N?
Fix N by forming symbiotic relationships with leguminous plants under microaerophilic conditions, where the bacteria utilize the enzyme nitrogenase to convert atmospheric N2 into ammonia (NH3), which the plants then use for growth.
what is Leghemoglobin?
A globin protein found in root nodules of leguminous plants eg peas, clover
Contains Fe-containing O2 binding protein and maintains a low & constant oxygen concentration within nodules, facilitating efficient N-fixation by rhizobia.
How are nodules formed in the root cells of legumes?
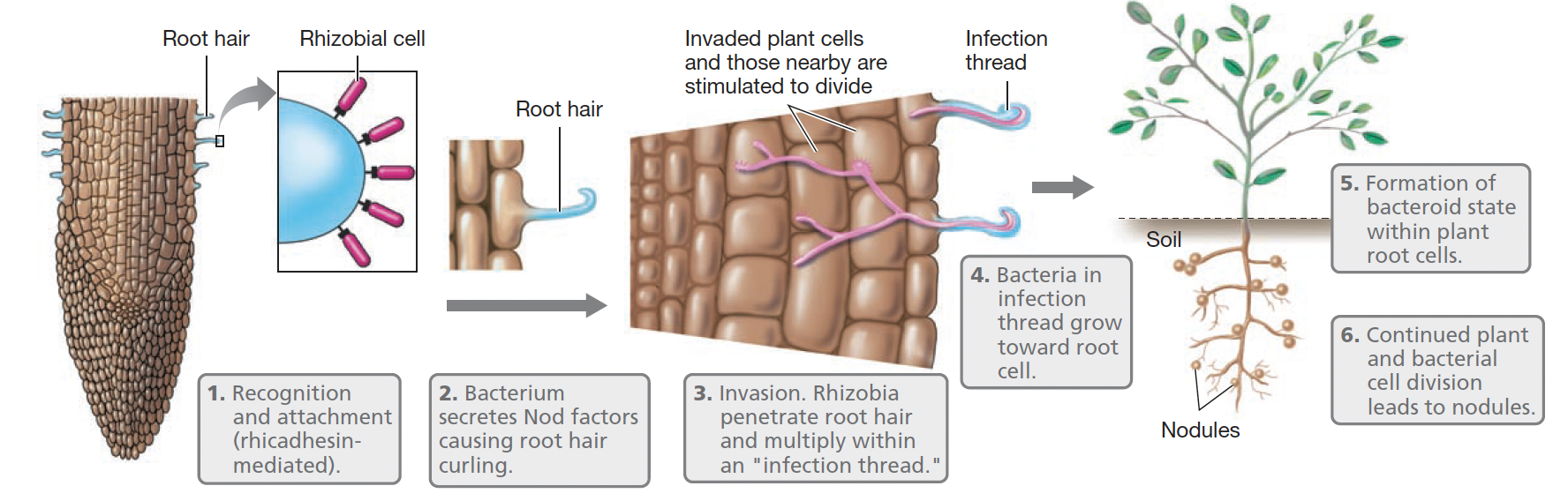
1. Attachment & Recognition of the bacterium to the root hairs
2. Secretion of oligosaccharide signalling molecules (Nod factors) by the bacterium
3. Bacterial invasion of the root hair
4. Movement of bacteria to the main root by way of the infection thread
5. Formation of modified bacterial cells (bacteroids) within the plant cells and development of the N2-fixing state
6. Continued plant and bacterial cell division, forming the mature root nodule
what is meant by metabolic co-operation / syntrophy?
Where two or more m/o combine metabolic capabilities to catabolise a substance that would not be catabolised by either alone
eg In Nitrosomonas and Nitrobacter (chemolithotrophic nitrifying bacteria)
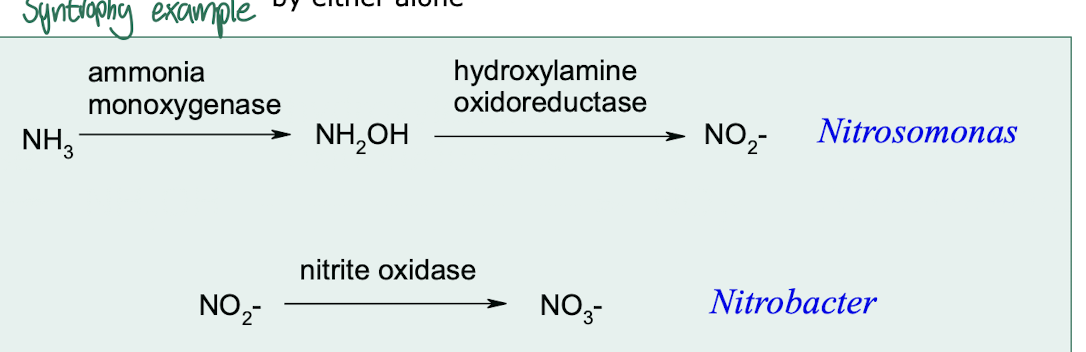
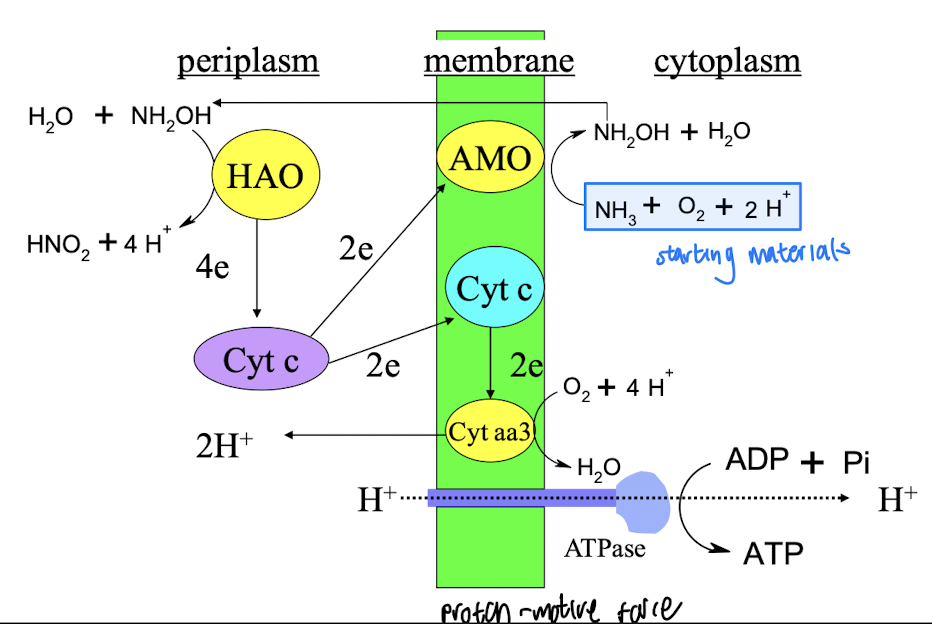
What enzymes are involved in nitrification within Nitrosomonas?
Enzymes such as ammonia monooxygenase (AMO) and hydroxylamine oxidoreductase, which are key in the conversion of ammonia to nitrite.
what m/o carry out denitrification?
Anaerobic organisms that convert nitrates into nitrogen gas, using NO3- as an e- acceptor & converting it into gaseous products thereby completing the nitrogen cycle.
Eg: Pseudomonas and Paracoccus species.
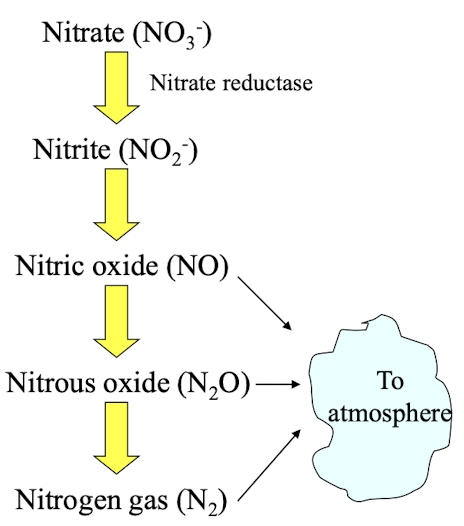
what are disadvantages + advantages of denitrification by m/o?
Disadvantage
Removes biologically available fixed N which is detrimental for agriculture
Advantage
Beneficial for sewage treatment by reducing nutrient pollution, preventing eutrophication in waterways by converting NO3− to volatile forms of N, denitrification minimises fixed N and thus algal growth
what are the 2 main oxidation states of Fe?
Fe2+ (ferrous)
Fe3+ (ferric)
what is the iron cycle?
Iron cycles primarily between the Fe2+ and Fe3+ forms, and these redox transitions are one-electron oxidations and reductions.
Ferric iron (Fe3+) is reduced both chemically and as a form of anaerobic respiration,
Ferrous iron(Fe2+) is oxidized both chemically and as a form of chemolithotrophic metabolism
*A 3rd oxidation state, Fe0, is abundant in Earth’s core & a major product of human activities from the smelting of iron ores to form cast iron
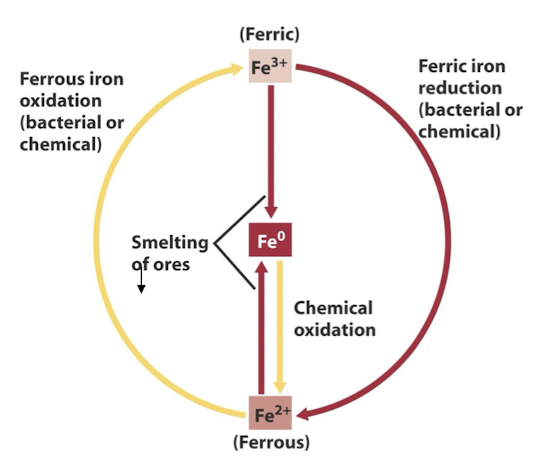
what are examples of iron oxidising bacteria ?
Acidithiobacillus ferrooxidans
Leptospirrilum ferrooxidans
They convert Fe2+ into Fe3+ under low pH conditions, aiding in biogeochemical cycling of iron.
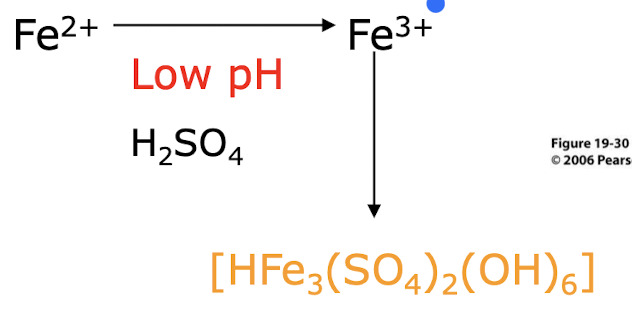
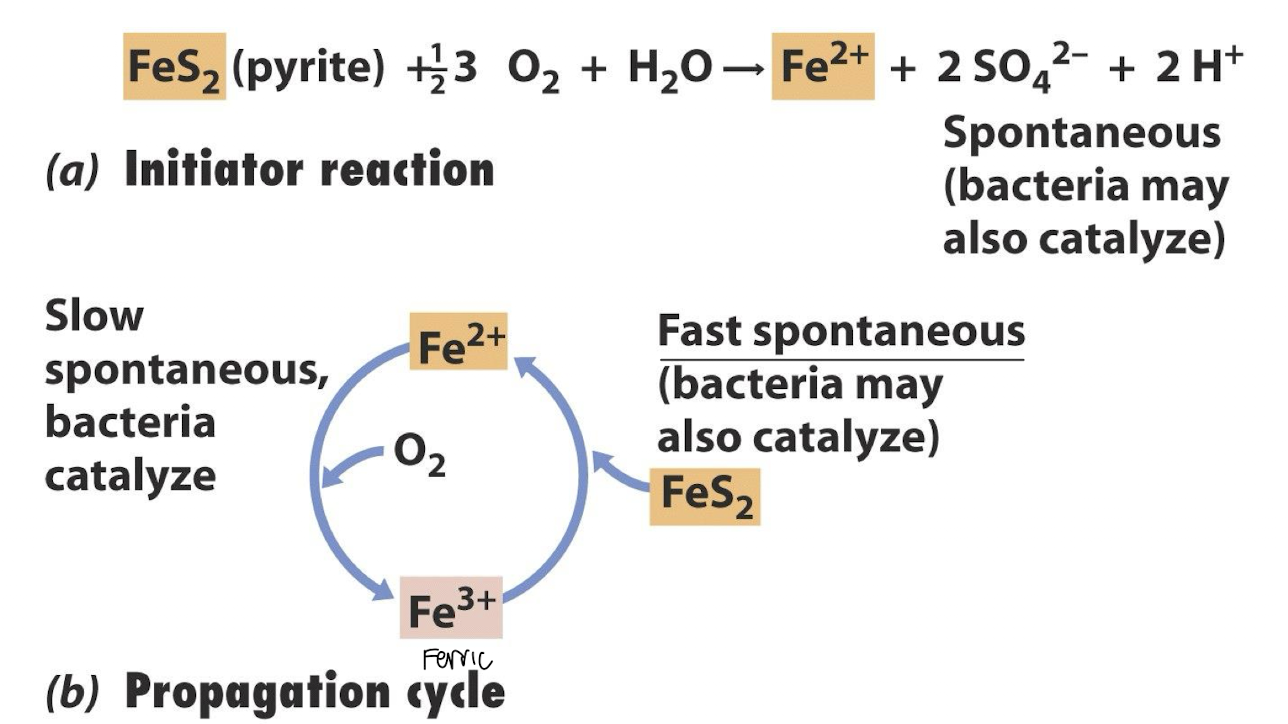
how is iron pyrite oxidised by bacteria?
Indirect microbial oxidation, where bacteria oxidize Fe²⁺ to Fe³⁺.
Fe³⁺Fe³⁺ is a strong oxidizer and reacts with pyrite to release more Fe²⁺ and sulfur compounds:
Sulfate and acid are released (toxic + makes water more acidic), fuelling a cycle that continues as long as oxygen, water, and pyrite are present.
Why does biological Fe2+ oxidation under oxic conditions occur mainly at acidic pH?
Biological Fe2+ oxidation is favored at acidic pH because low pH enhances the solubility of iron and promotes the activity of iron-oxidizing bacteria, which thrive in acidic environments.
what is Acid Mine drainage?
Outflow of Acidic water containing H2SO4 (sulfuric acid) derived from the microbial oxidation of iron sulfide (FeS2) minerals eg Pyrite
It can lead to the contamination of surrounding water bodies, harming aquatic life and ecosystems by the solublisation of heavy metals eg Al
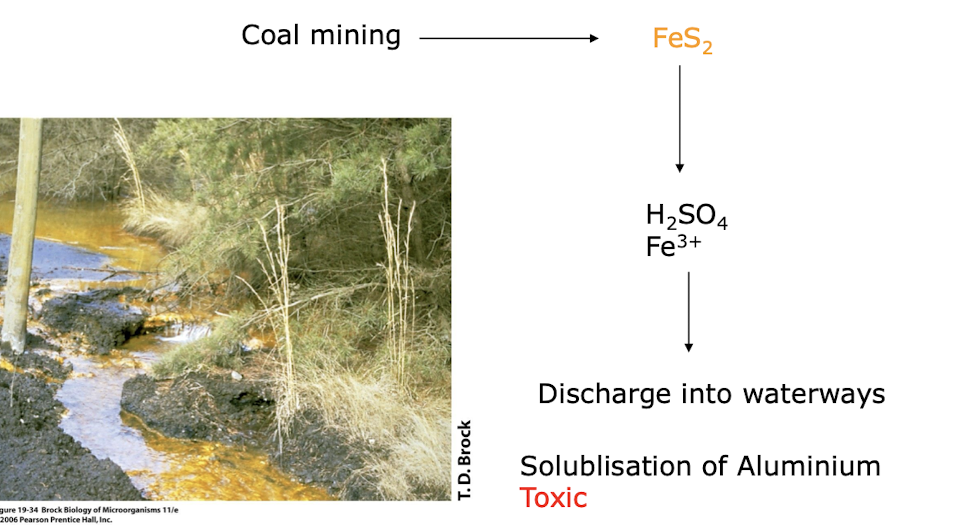
why is Ferroplasma acidiphilium an interesting m/o?
Strong acidophile: can live in environments of pH < 0, such as those found in acid mine drainage.
Oxidises ferrous iron (Fe2+) to ferric iron (Fe3+) to obtain energy (this rxn generates acid and uses CO2 as its carbon source (autotrophy).
Grows in mine tailings containing pyrite (FeS2), which is its energy source.
The extreme acidophily of Ferroplasma allows it to drive the pH of its habitat down to extremely acidic values.