vibrational/infrared spectroscopy
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
what does vibrational spectroscopy probe
molecular motions due to movement of atoms in individual chemical bonds
EM region for vibrational spectroscopy and what it is used for
IR region
used to identify functional groups in molecules, confirm the structure of compounds and measure the vibrational frequencies and force constants of chemical bonds
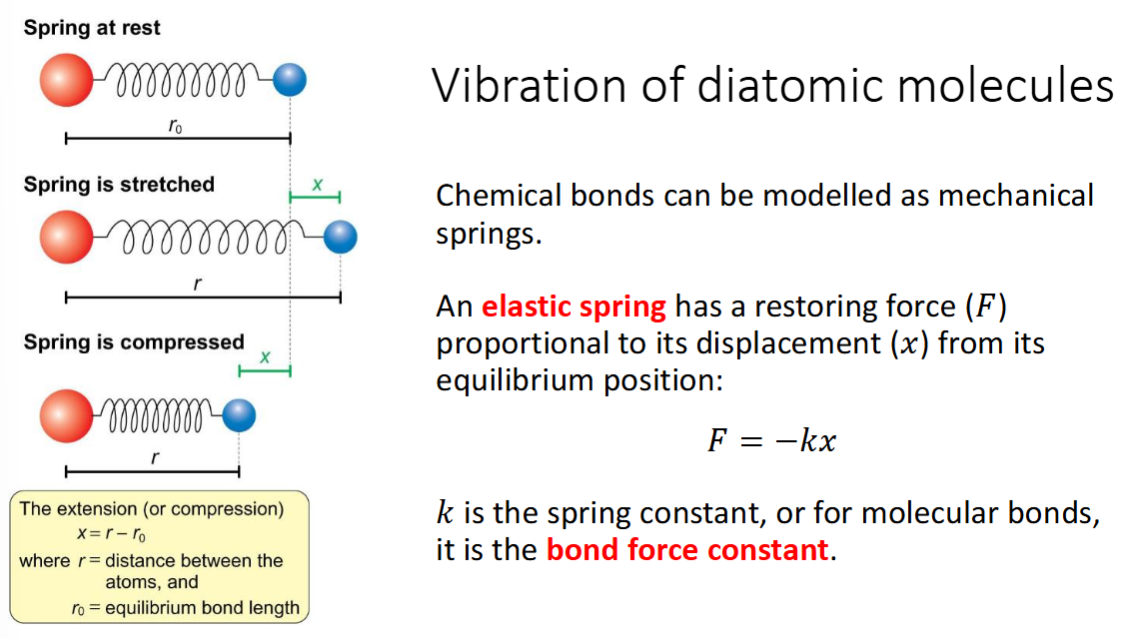
vibration of diatomic molecules
how is it modelled
equation?
diagram (3 scenarios)
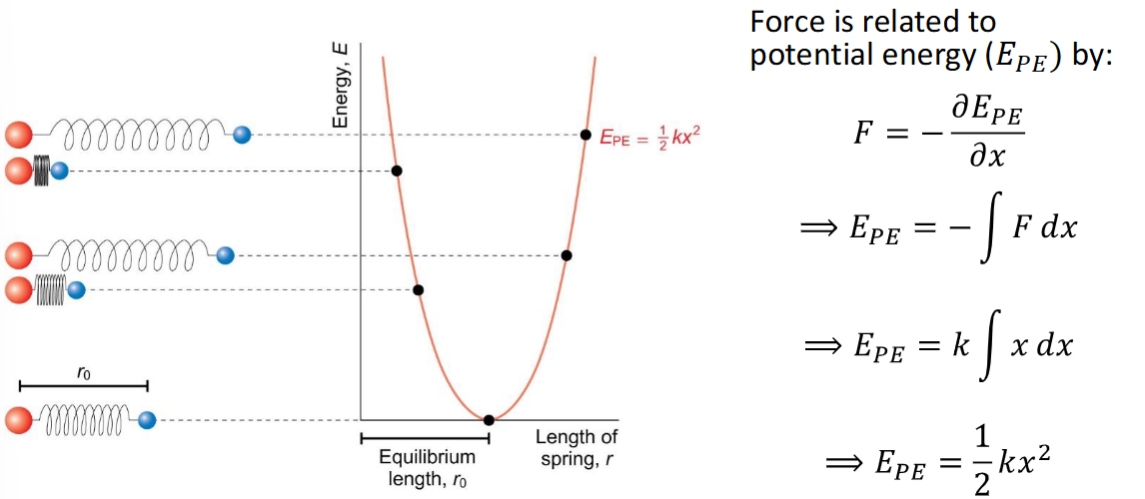
how are potential energy and force related
equation + derviation
graph/diagram
vibrational frequency of a diatomic molecule
the bond oscillates, undergoing simple harmonic motion
the vibrational frequency depends on
mass of atoms - heavier atoms vibrate at a slower frequency
bond force constant - stiffer “springs” vibrate at higher frequency
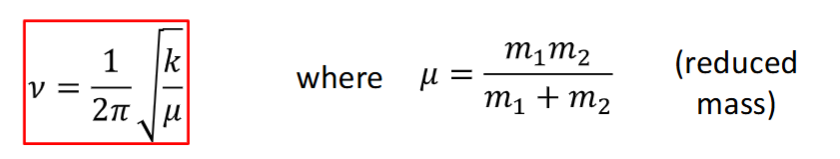
how is the equation for EV found
what is it and what are the terms
found by solving schrodinger’s equation with V(x) = ½kx2
v is the vibrational quantum number which takes integer values v ≥ 0
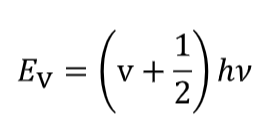
ground state for vibrational energy?
there is a finite ground state energy:
EV=0 = ½hv - as lowest v=0 → vibrational does not allow zero energy level
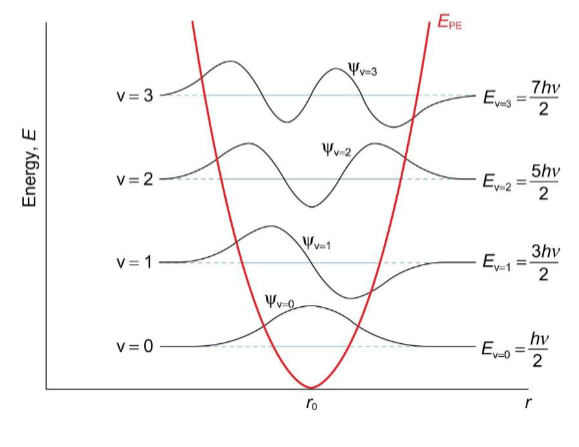
vibrational energy levels and wavefunctions
wavefunctions are finite outside of the parabolic potential well - an example of quantum mechanical tunnelling
classically, the particle is forbidden to exist outside the potential well, but there is a finite probability of the particle penetrating this region in quantum mechanics - it continues outside the red line for a short period
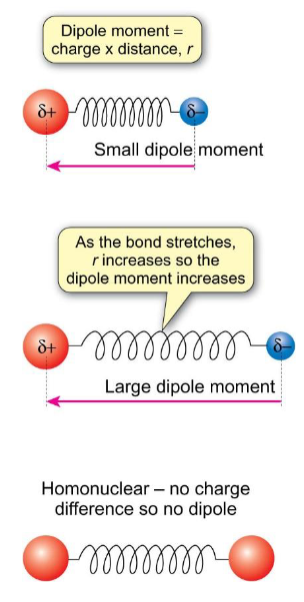
gross selection rule for vibrational
there must be a change in the dipole moment of the molecule during the vibration - for diatomics, this means the molecule must have a permanent dipole moment. homonuclear diatomics eg H2 do not give IR spectra
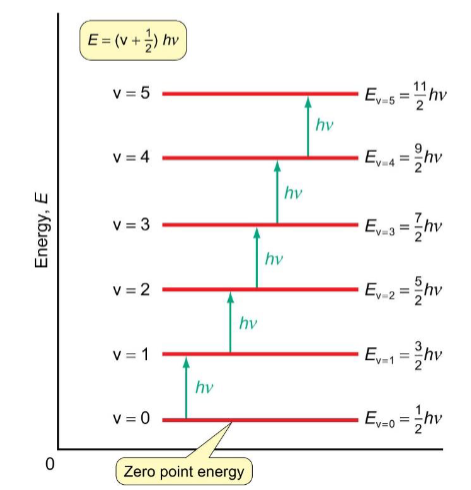
specific selection rule for vibrational
Δv = ±1
+1 for absorption and -1 for emission spectra
vibrational spectra of diatomic molecules
transitions are between adjacent levels - all ΔE = hv
vibrational spectroscopy can only probe the transitions and the gaps are identical for diatomics, so only one peak is expected in the spectrum
how are bond force constants measured
force constant is a measure of stiffness and therefore of strength
combine equations for Ev and v to give this equation
calculate reduced mass
k can be calculated from the equation for ΔE
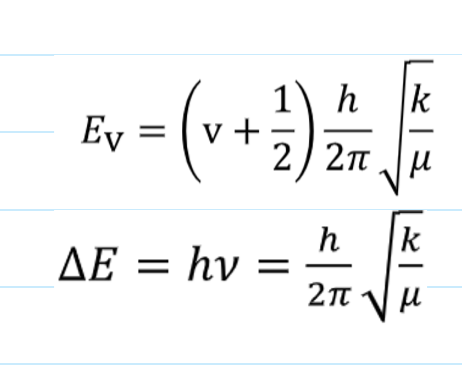
populations of vibrational energy levels
use Botlzmann distribution again
in this case the ground state has approx 2 million times the occupancy of higher states (can usually expect this as expect molecules to be in ground state)
this means the dominant transition, and the one causing the spectrum, is v=0 → v=1 (as can only move out of v=0 and the specific selection rule means can only go to v=1)
effect of isotopic substitution
when is it most significant
the bond force constant is a property of the chemical bond and therefore the electron distribution
this is not expected to change significantly by adding extra neutrons to the nucleus - ie k is assumed to be independent of the isotope
there are slight shifts in spectra, and heavier isotopes cause lower frequencies
the shift in spectra is most significant for H → D isotopic substitution as the lightest atom moves much more than the heavy atom and dominates the value of the reduced mass.
anharmonic vibrations
when used
equation (name?)
graph
assumption of harmonic oscillations is good but not exact. real molecules undergo anharmonic oscillations - energy levels are still in good agreement for low v.
Morse curve/Morse potential
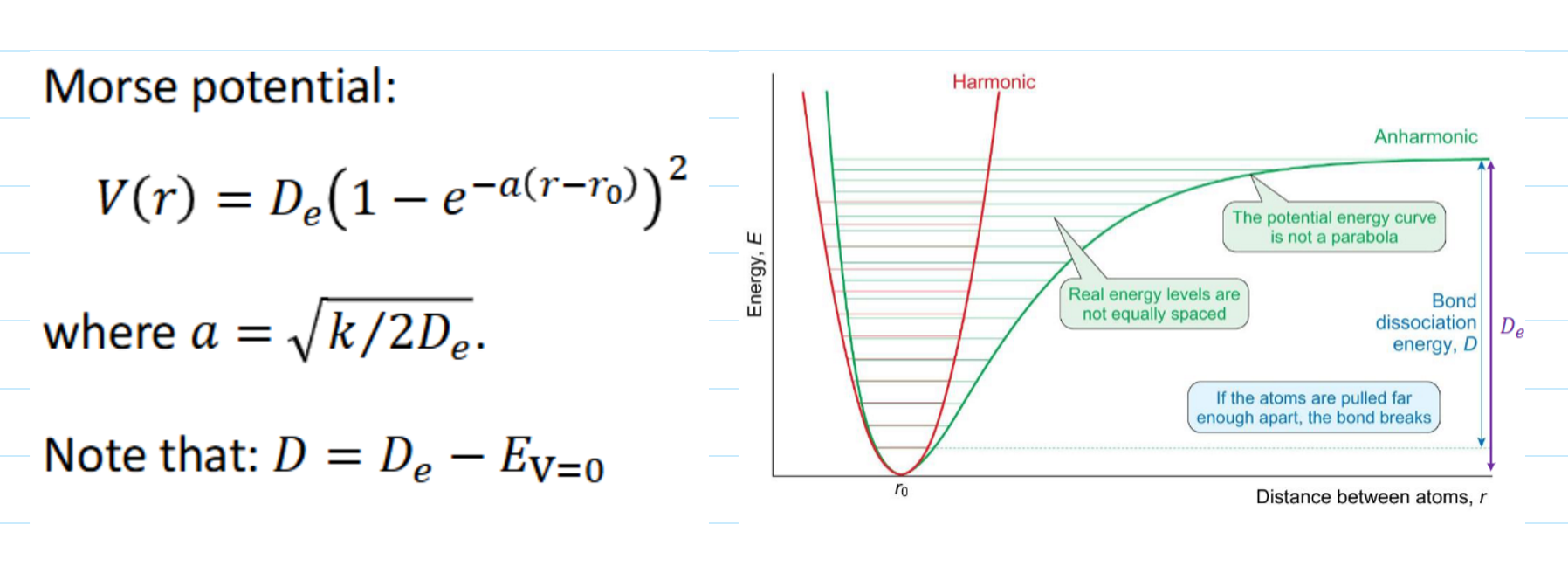
energy levels for anharmonic oscillators
what happens as v increases
ΔE gets smaller as v increases
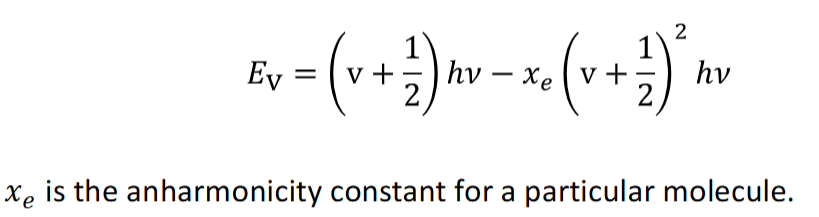
what are overtones
the selection rule Δv = ±1 is strictly for harmonic oscillators
for anharmonic oscillations in real molecules, transitions v=0 → v=2 and v=0 → v=3 are observed. these are called overtone bands and occur at twice and three times the fundamental frequency - however they are much weaker than the fundamental absorption v=0 → v=1
polyatomic molecules
there are vibrational modes due to bending (changes in bond angle) as well as symmetric and asymmetric stretches
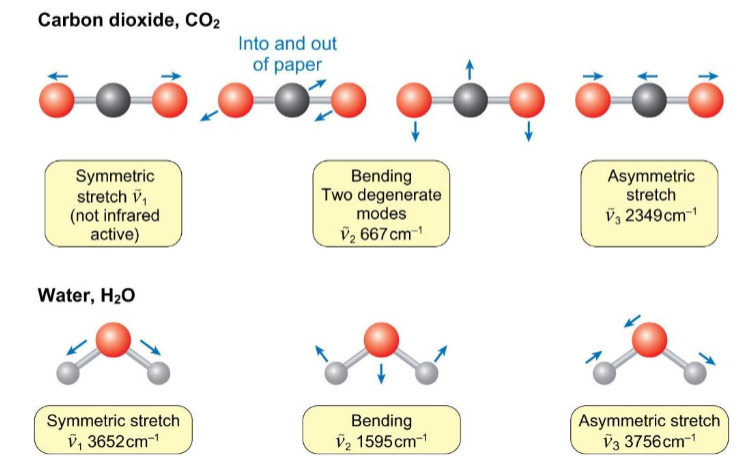
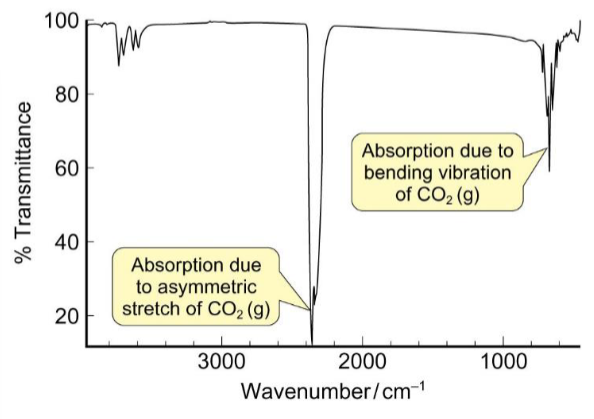
how are atmospheric concentrations of CO2 measured
the symmetric stretch of CO2 does not change the dipole moment so its not IR-active
the absorption of the asymmetric stretch can be used for atmospheric measurement of CO2 concentrations
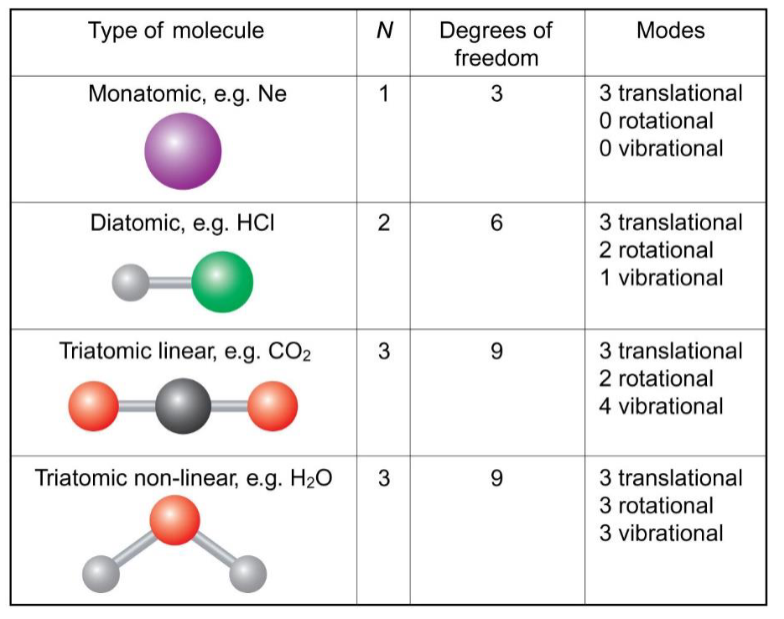
vibrational modes of N atom molecules
more atoms = more possible modes of vibration
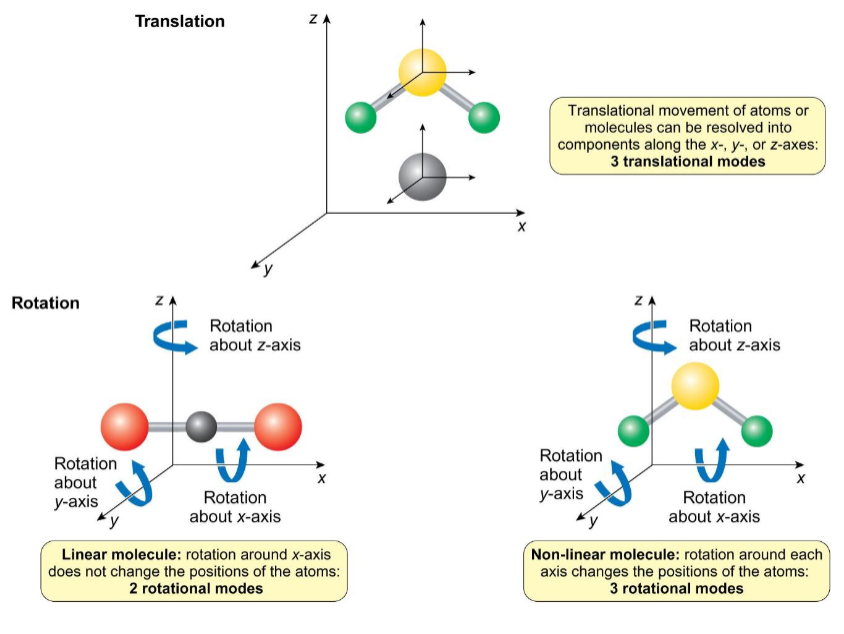
linear molecules have (3N-5) vibrational modes
non-linear molecules have (3N-6) vibrational modes
each vibrational mode has its own potential energy curve and series of vibrational energy levels
degrees of freedom
N atoms have 3N degrees of freedom
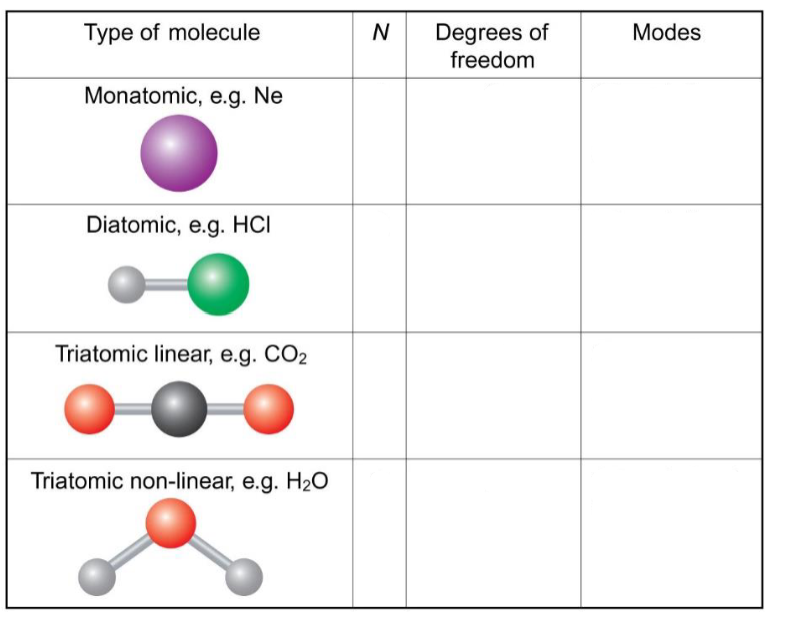
complete