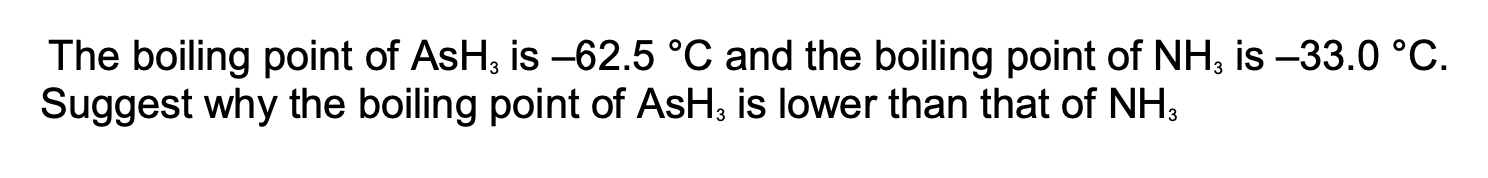A level Chemistry - Bonding
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
45 Terms
What is ionic bonding?
Ionic bonding is the electrostatic force of attraction between oppositely charged ions formed by electron transfer.
occurs between a metal and a non metal
Metal atoms lose electrons to form +ve ions. Non-metal atoms gain electrons to form -ve ions.
structure: giant ionic structure
when is ionic bonding stronger?
Ionic bonding is stronger and the melting points higher when the ions are smaller and/ or have higher charges. E.g. MgO has a higher melting point than NaCl as the ions involved (Mg2+ & O2- are smaller and have higher charges than those in NaCl , Na+ & Cl-
Explain what affects the size of ionic radii
Positive ions are smaller compared to their atoms because they have one less shell of electrons and the ratio of protons to electrons has increased so there is greater net force on remaining electrons holding them more closely.
The negative ions formed from groups five to seven are larger than the corresponding atoms.
The negative ion has more electrons than the corresponding atom but the same number of protons. So the pull of the nucleus is shared over more electrons and the attraction per electron is less, making the ion bigger.
Within a group the size of the ionic radii increases going down the group. This is because as one goes down the group the ions have more shells of electrons.
What is a covalent bond?
A covalent bond is a shared pair of electrons
What is dative covalent/ coordinate bonding?
A dative covalent bond forms when the
shared pair of electrons in the covalent bond
come from only one of the bonding atoms
What is metallic bonding?
Metallic bonding is the electrostatic force of attraction between the positive metal ions and the delocalised electrons
What factors affect the strength of a metallic bond?
charge on the metal ions
The higher the charge the stronger the bond
2. Number of delocalised electrons per atom (the outer shell electrons are delocalised)
The more delocalised electrons the stronger the bond
3. Size of ion.
The smaller the ion, the stronger the bond.
Explain the properties and structure of simple covalent substances
examples: Iodine ,Ice ,Carbon dioxide, Water, Methane
boiling/melting point : low- because of weak intermolecular forces between molecules (specify type e.g van der waals/hydrogen bond)
solubility: poor
conductivity: poor: no ions to conduct and electrons are localised (fixed in place)
generally: mostly gases and liquids
explain the properties and structure of ionic substances
Melting/ Boiling point: high- because of giant lattice of ions with strong electrostatic forces between oppositely charged ions.
soluble in water
conductivity: only when molten or dissolved, ions are free to move and carry charge through the structure
generally: crystalline solids
giant ionic lattice structure , regular and repeating
What are the key macromolecular / giant covalent structures?
Diamond, Graphite , Silicon, Silicon dioxide
explain the structure and properties of diamond
Giant covalent lattice
Each C bonded to 4 other C atoms in a tetrahedral shape
Strong covalent bonds throughout the lattice
Properties:
Property | Reason |
|---|---|
Very hard | Each atom strongly bonded to 4 others |
Very high melting/boiling point | Many strong covalent bonds must be broken |
Does not conduct electricity | No free electrons or ions |
Insoluble in water | Covalent bonds too strong to break with solvents |
explain the structure and properties of graphite
Structure:
Giant covalent lattice
Forms hexagonal layers of graphene
Weak van der Waals forces between layers
Each carbon has one delocalized electron per atom
Properties:
Property | Reason |
|---|---|
Soft/slippery | Layers slide over each other (weak forces) |
High melting point | Covalent bonds within layers are strong |
Conducts electricity | Delocalized electrons can move along layers |
Insoluble in water | Covalent bonds too strong |
Explain the structure and properties of silicon dioxide
Structure:
Giant covalent lattice
Each silicon atom bonded to 4 oxygen atoms (tetrahedral)
Each oxygen bonded to 2 silicon atoms
Repeating 3D structure
Properties:
Property | Reason |
|---|---|
Very hard | Strong Si–O covalent bonds |
Very high melting/boiling point | Many bonds to break |
Does not conduct electricity | No free electrons or ions |
Insoluble in water | Strong covalent lattice |
Explain the structure and properties of silicon
Structure:
Giant covalent lattice (like diamond)
Each Si bonded to 4 other Si atoms tetrahedrally
Properties:
Property | Reason |
|---|---|
Hard | Covalent bonds throughout lattice |
High melting point | Strong covalent bonds |
Semi-conductor | Can conduct electricity slightly under certain conditions |
Insoluble in water | Covalent bonds too strong |
SHAPES OF MOLECULES LEARN ON PAPER :)
.
What is electronegativity?
The power of an atom or nucleus to withdraw or attract electrons → MS
Electronegativity is the relative tendency of an atom in a covalent bond in a molecule to attract electrons in a covalent bond to itself.
what are the most electronegative atoms?
F, O, N and Cl are the most electronegative atoms
The most electronegative element is fluorine and it is given a value of 4.0
what is electronegativity measured with?
Electronegativity is measured on the Pauling scale (ranges from 0 to 4)
What factors affect electronegativity?
Electronegativity increases across a period as the number of protons increases and the atomic radius decreases because the electrons in the same shell are pulled in more.
It decreases down a group because the distance between the nucleus and the outer electrons increases and the shielding of inner shell electrons increases
How can differences in electronegativity determine whether compounds are covalent/ ionic bonds?
A compound containing elements of similar electronegativity and hence a small electronegativity difference will be purely covalent
A compound containing elements of very different electronegativity and hence a very large electronegativity difference (> 1.7) will be ionic
What is a permanent dipole/ polar covalent bond?
A polar covalent bond forms when the elements in the bond have different electronegativities . (Of around 0.3 to 1.7)
When a bond is a polar covalent bond it has an unequal distribution of electrons in the bond and produces a charge separation, (dipole) δ+ δ- ends.
The element with the larger electronegativity in a polar compound will be the δ- end.
What is a symmetric molecule?
when all bonds identical and no lone pairs
will a symmetric molecule be polar?
No, it will not be polar even if individual bonds within the molecular are polar.
this is because the individual dipoles on the bonds ‘cancel out’ due to the symmetrical shape of the molecule. There is no net dipole moment: the molecule is non-polar
Describe induced dipole-dipole forces ( Van Der Waals/ dispersion/ London forces )
They occur between all simple covalent molecules and the separate atoms in noble gases.
They do not occur in ionic substances.
- In any molecule the electrons are moving constantly and randomly. As this happens the electron density can fluctuate and parts of the molecule become more or less negative i.e. small temporary or transient dipoles form.These instantaneous dipoles can cause dipoles to form in neighbouring molecules. These are called induced dipoles. The induced dipole is always the opposite sign to the original one. A temporary dipole forms between the ∂+ in one molecule and the ∂- in a neighbouring molecule.
What factor affects the size of Vaan Der Waals forces?
The more electrons there are in the molecule the higher the chance that temporary dipoles will form. This makes the Van der Waals stronger between the molecules and so boiling points will be greater.
-The shape of the molecule can also have an effect on the size of the Van der Waals forces. Long chain alkanes have a larger surface area of contact between molecules for Van der Waals to form than compared to spherical shaped branched alkanes and so have stronger Van der Waals.
this explains increasing boiling points down group 7 and of the alkane homologous series
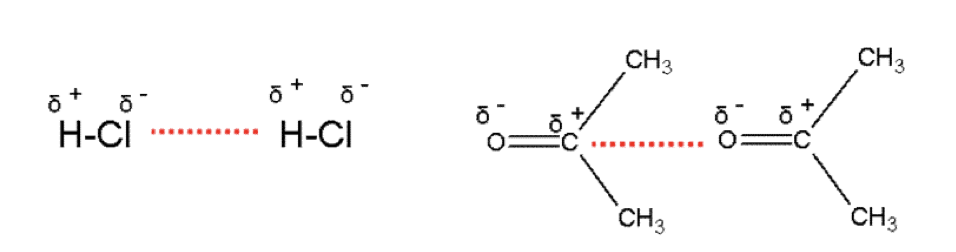
Describe permeant dipole-dipole forces
•Permanent dipole-dipole forces occurs between polar molecules
•They are stronger than Van der Waals and so the compounds have higher boiling points
•Polar molecules have a permanent dipole. (commonly compounds with C-Cl, C-F, C-Br H-Cl, C=O bonds)
•Polar molecules are asymmetrical and have a bond where there is a significant difference in electronegativity between the atoms.
Permanent dipole-dipole forces occurs in addition to Van der Waals forces
Descrive hydrogen bonding
Definition: A hydrogen bond is a strong intermolecular attraction between a δ⁺ hydrogen and a lone pair on N, O, or F in a neighbouring molecule.
How it forms:
H is covalently bonded to N, O, or F → H becomes δ⁺.
A nearby molecule has N, O, or F with a lone pair (δ⁻).
The δ⁺ H is attracted to the δ⁻ lone pair → hydrogen bond forms.
Key point: δ⁺ H forms first within its own molecule, lone pair only matters when bonding to another molecule.
strongest type of dipole-dipole interaction
Hydrogen bonding occurs in addition to van der waals forces
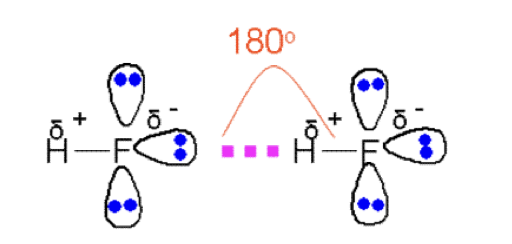
can you explain the importance of hydrogen bonding in the low density of ice?
Ice is solid water (H₂O).
Each water molecule forms four hydrogen bonds with neighboring molecules.
This forms a rigid, open, tetrahedral lattice.
Key Points:
The lattice holds water molecules further apart than in liquid water.
Liquid water has fewer hydrogen bonds and molecules are closer together.
Therefore, ice is less dense than water → it floats.
can you explain the importance of hydrogen bonding in the anomalous boiling points of compounds?
Normally, boiling point increases with molecular mass due to stronger van der Waals forces.
However, H₂O, NH₃, HF have higher boiling points than expected.
Why:
They have hydrogen bonding in addition to van der Waals forces.
Extra energy is required to break hydrogen bonds when boiling.
can you explain how melting/boiling points are influenced by intermolecular forces? and how to tell which forces are present in unfamiliar molecules?
Force | Present in | Strength | Effect on BP/MP |
|---|---|---|---|
Van der Waals | All molecules | Weak | Increases with size |
Permanent dipole–dipole | Polar molecules | Medium | Higher BP than non-polar of similar size |
Hydrogen bonding | H bonded to N/O/F | Strong | Much higher BP/MP, affects solubility, ice density |
What shape and bond angle do you have with two bonding pairs and no lone pairs ?
linear
angle: 180
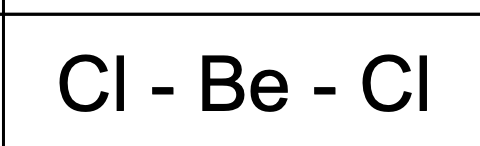
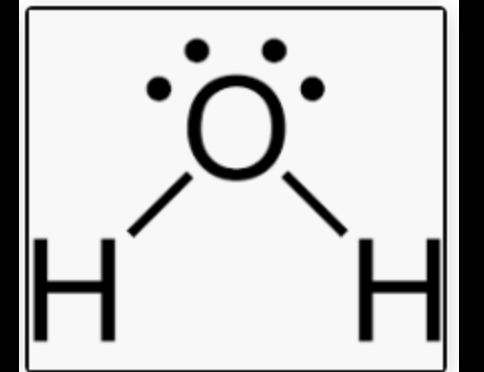
What shape and bond angle do you have with 2 bonding pairs and 2 lone pairs ?
V- shaped
angle: 104.5
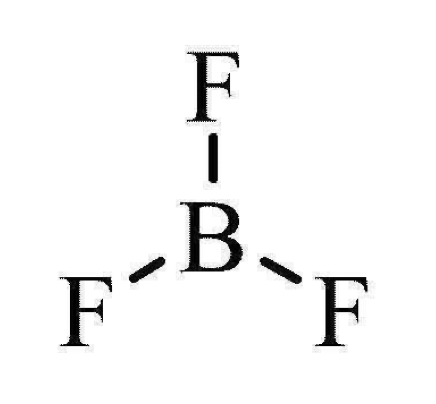
What shape and bond angle do you have with 3 bonding pairs and no lone pairs ?
trigonal planar
angle: 120
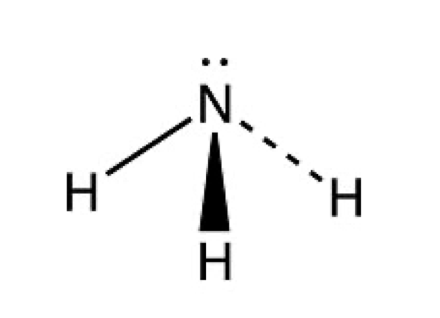
What shape and bond angle do you have with 3 bonding pairs and 1 lone pair ?
trigonal pyramid
angle: 107
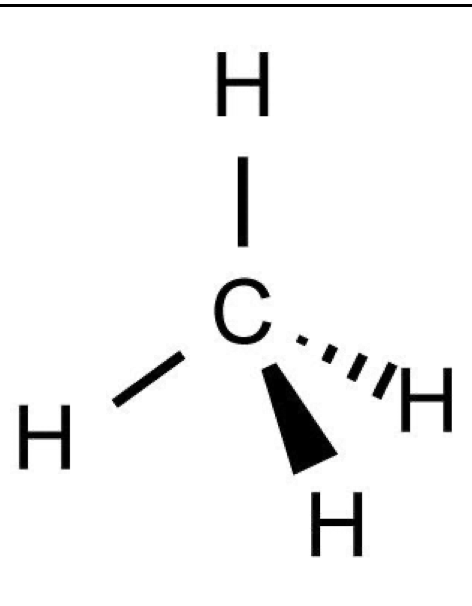
What shape and bond angle do you have with 4 bonding pairs and no lone pairs ?
teterahederal
angle: 109.5
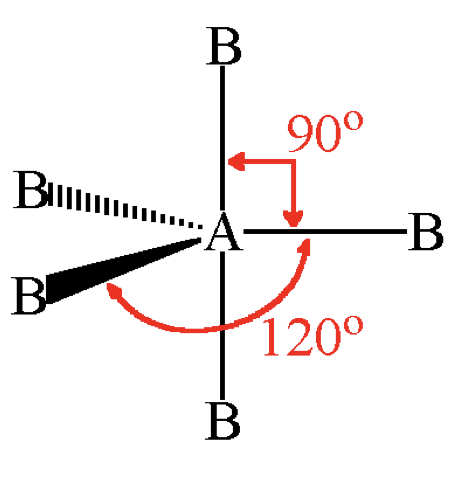
What shape and bond angle do you have with 5 bonding pairs and no lone pairs ?
trigonal bypyramid
angles: 90 and 120
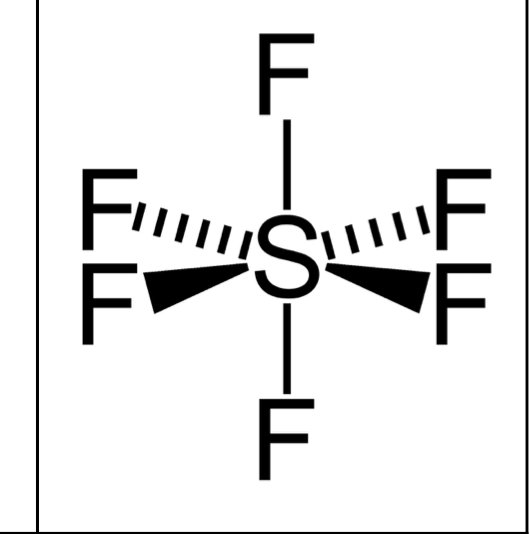
What shape and bond angle do you have with 6 bonding pairs and no lone pairs ?
octrahederal
angle: 90
Explain, in terms of electronegativty , why the boiling point of H2S2 is lower than H2O2
Electronegativity of S lower than O or electronegativity difference between H and S is lower
No hydrogen bonding between H2S2 molecules

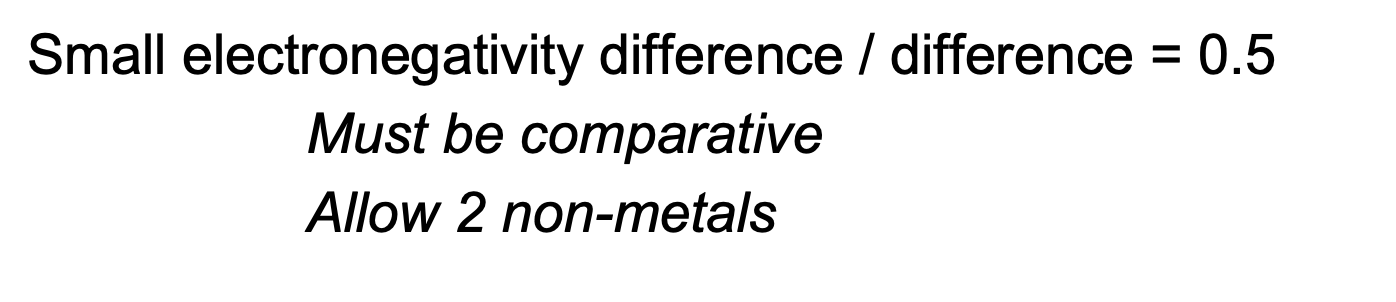





my answer: there is no O, N or F presnt → be more specific