L2
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
What is the basic structural organisation of all myosins?
One or two heavy chains forming the motor (head) domain plus several light chains bound to the neck region.
What is the structure of myosin II ?
two head domains and two light chains per neck
only class that assemble into bipolarfilaments
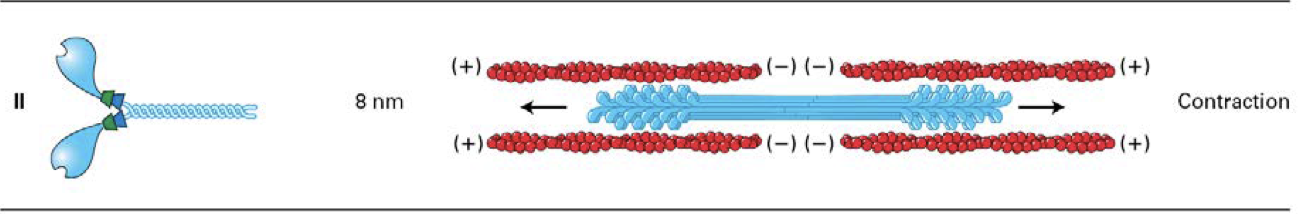
What is the structure of myosin I ?
the only myosins to have a single head domain
some of these myosins associate directly with membranes through lipid interactions
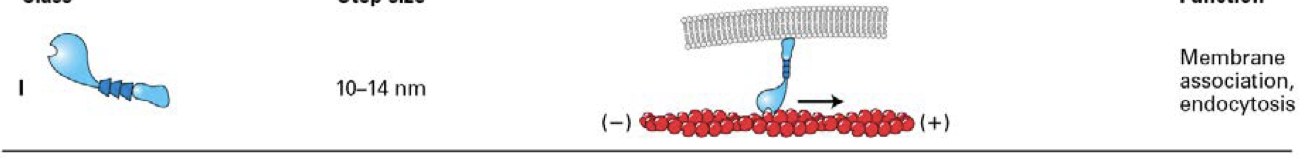
what is the structure of myosin V ?
two head domains and six light chains per neck.
Bind specific receptors (brown box) on organelles, which they transport
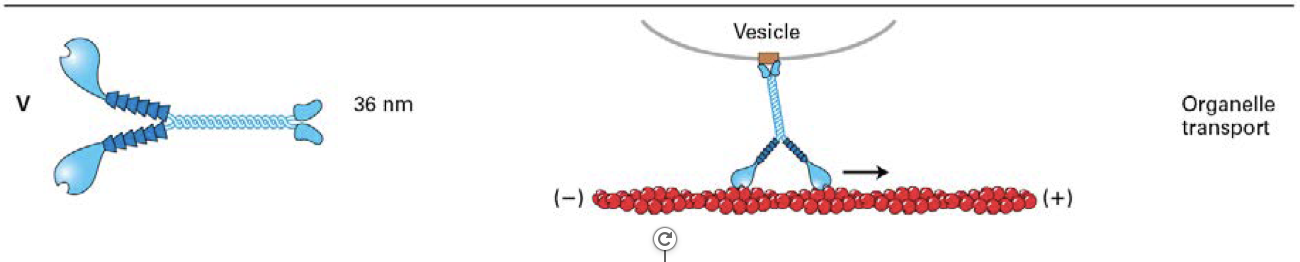
what do all of these myosin classes have in common?
move toward the (+) end of actin filaments
- only myosin VI moves (cargo) towards (-) end of an actin filament
What functions are performed by the myosin head?
Binds actin
binds and hydrolyses ATP
generates force
explain the ATP-driven myosin movement along actin filaments
When ATP binds to myosin, it replaces ADP, the ATP then causes the myosin head to be released from the actin filament
Upon ATP hydrolysis into ADP + Pi (which remain bound) , the myosin head undergoes a conformational change that rotates the head with respect to the neck portion of the protein - “cocked state”
Myosin-ADP-Pi complex binds a new actin subunit
Pi is released and the myosin head moves back to its original conformation, moving the bound actin filament - “power stroke”
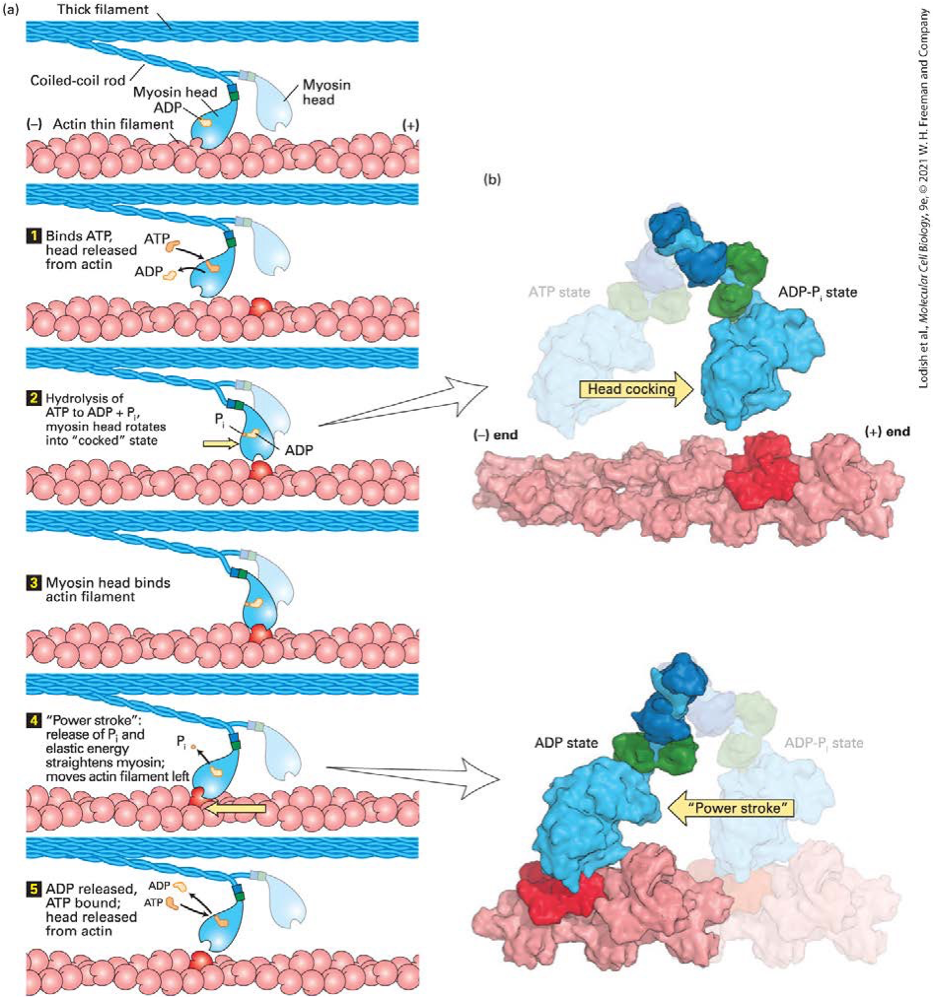
How is hydrolysis of ATP in the nucleotide-binding pocket of the head converted into a mechanical force?
Hydrolysis of ATP induces a small conformational change in the head domain
This small movement is amplified by a converter region at the base of the head, which acts like a fulcrum causing the rodlike neck (also known as the lever arm) to rotate
The rotation is amplified by the neck domain, so the actin filament moves by a few nanometers
The distance a myosin moves along actin during hydrolysis of one ATP — the myosin step size — is proportional to the length of the neck domain
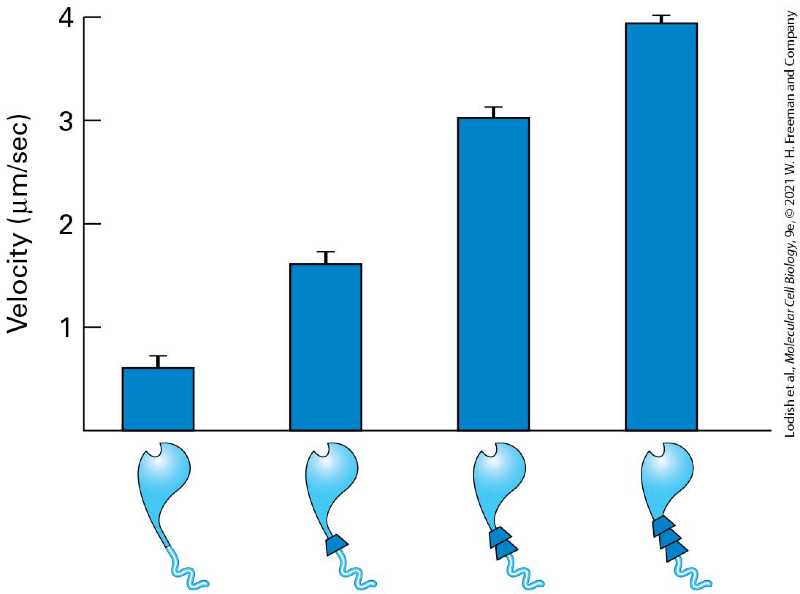
how does the length of the myosin II neck domain determines the rate of movement?
The rate at which these myosins moved on actin filaments reflects the myosin step size - the longer the lever arm, the faster the myosin was observed to move
increase in neck size increase in neck size so the faster the myosin is able to move along the microfilament
what are the main structural differences between myosin II and myosin V explain
Myosin V:
- longer neck
- globular cargo binding domains at its tail
Myosin II :
- assemble into bipolar filaments involved in contractile functions
How do myosin tail domains differ between classes?
Tail domains vary and determine myosin function, cargo binding, or filament assembly
What determines myosin step size?
Length of the neck (lever arm) domain
How does myosin V move along actin?
Hand-over-hand movement with large step size (~36 nm).
How is myosin V activity regulated?
Inactive folded state; cargo binding unfolds and activates the motor.
Why is myosin II suited for contraction rather than transport?
Short duty cycle and cooperative action of many motors
What non-muscle cellular processes require myosin II?
Stress fibres
adherens belts
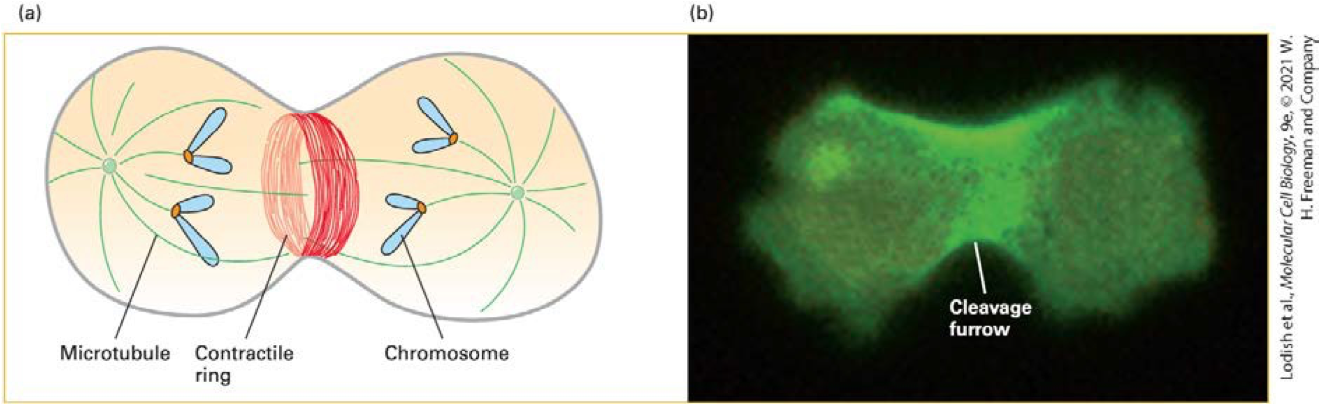
cytokinesis (contractile ring)
What happens when myosin II is deleted from dividing cells?
Cytokinesis fails, producing multinucleated cells
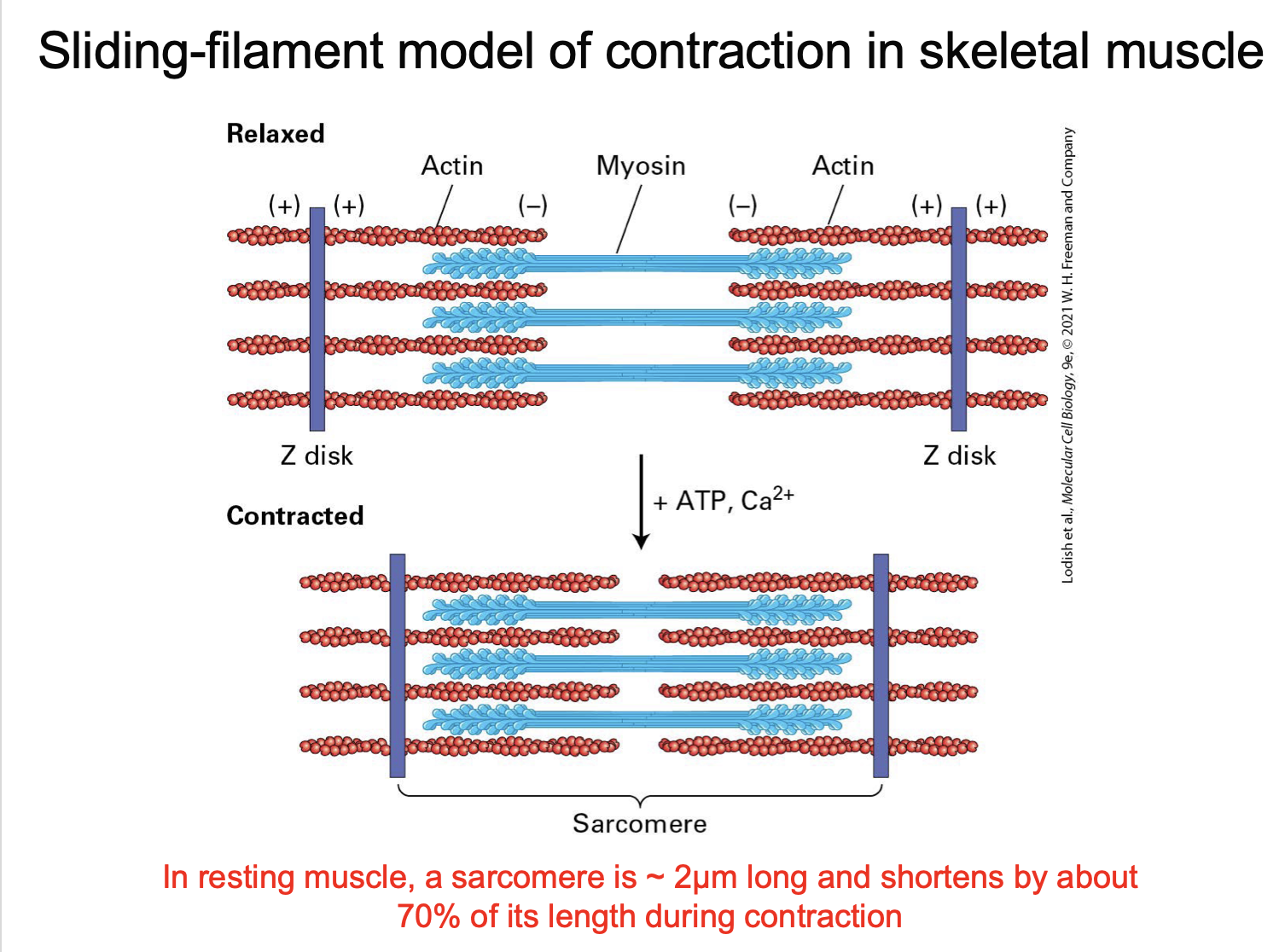
What is a sarcomere?
The basic contractile unit of a muscle fibre - stetches from one Z disc to another
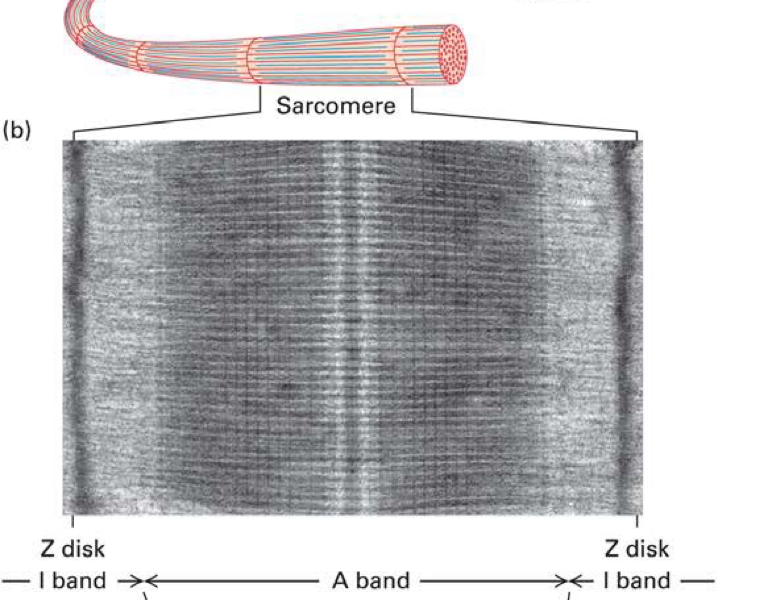
what is the A band?
the whole myosin
there is also some overlap with the actin
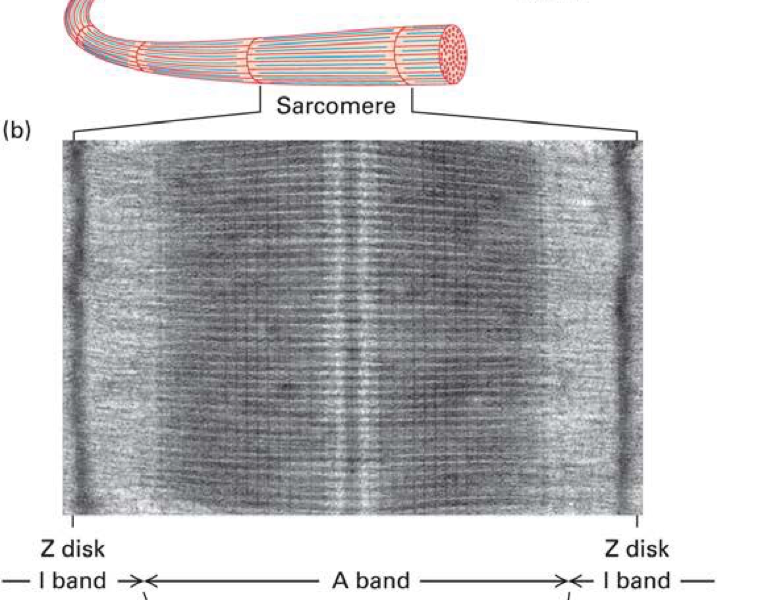
What is the z disc
defines the end of the sarcomere
What is the I band?
actin only
How does the sliding-filament model explain contraction?
Myosin pulls actin toward the centre, shortening the sarcomere
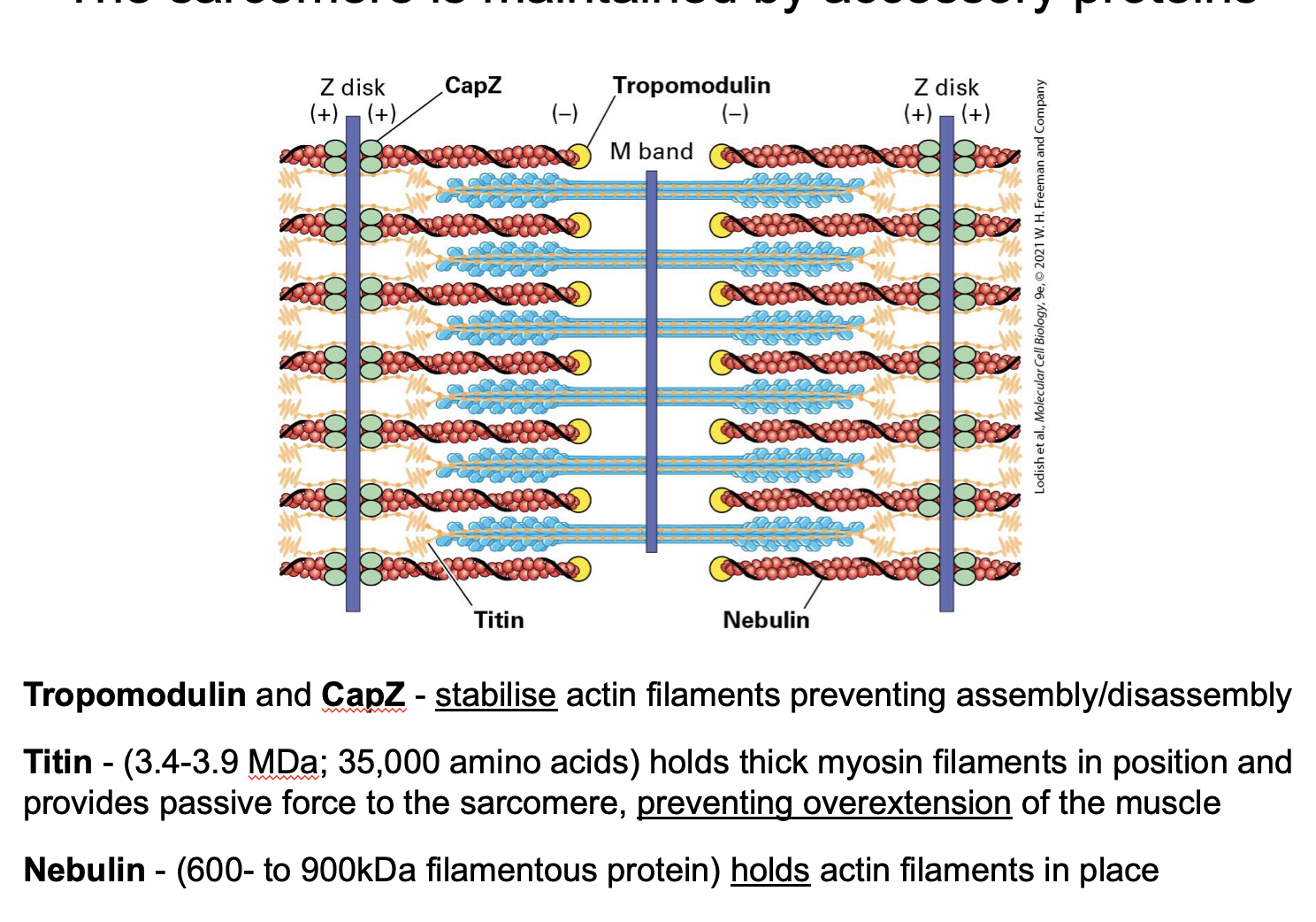
What proteins stabilise the sarcomere?
Titin
nebulin
CapZ
tropomodulin
What triggers skeletal muscle contraction?
Increase in cytosolic Ca²⁺ concentration
How does Ca²⁺ allow myosin to bind actin?
Ca²⁺ binds troponin
shifting tropomyosin to expose myosin-binding sites
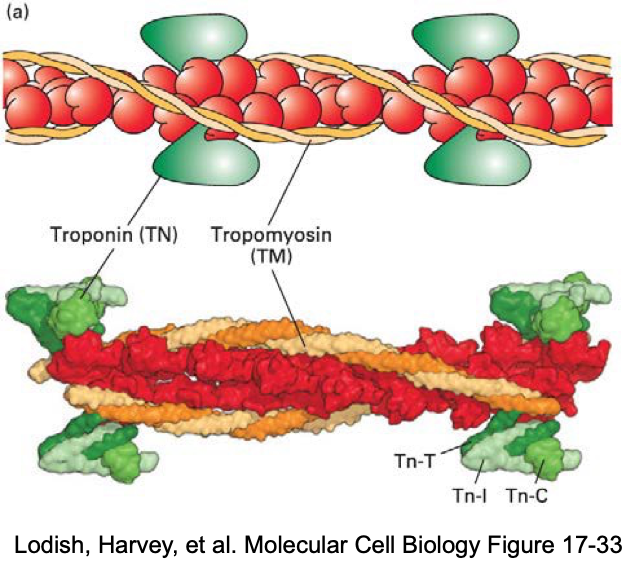
what is tropomyosin?
rope like molecule
binds to 7 actin subunits
continuous chain
Associated with each tropomyosin molecule is troponin molecule, a complex of 3 subunits, TN-T, TN-I, and TN-C → which control the position of the tropomyosin on the actin filaments under the presence of Ca2+
What happens when Ca²⁺ is removed from the cytosol and stored in the SR?
tropomyosin blocks binding sites and contraction stops
How does smooth muscle regulation differ from skeletal muscle?
smooth muscle regulation occurs at the myosin (thick filament) level, not actin
What role does Ca²⁺ play in smooth muscle contraction?
Ca²⁺ binds calmodulin
activating myosin light-chain kinase
What is the role of myosin light-chain phosphorylation?
Phosphorylation activates myosin II and promotes filament assembly
How does smooth muscle relax?
Myosin light-chain phosphatase dephosphorylates myosin
Why is smooth muscle contraction slower than skeletal muscle?
Thick-filament regulation relies on kinase activity and Ca²⁺ diffusion
What types of signals regulate smooth and non-muscle contraction?
Hormones
neurotransmitters
immune mediators
signalling pathways
what are hypertrophic cardiomyopathies?
thickening of the heart wall muscle
which compromises its function
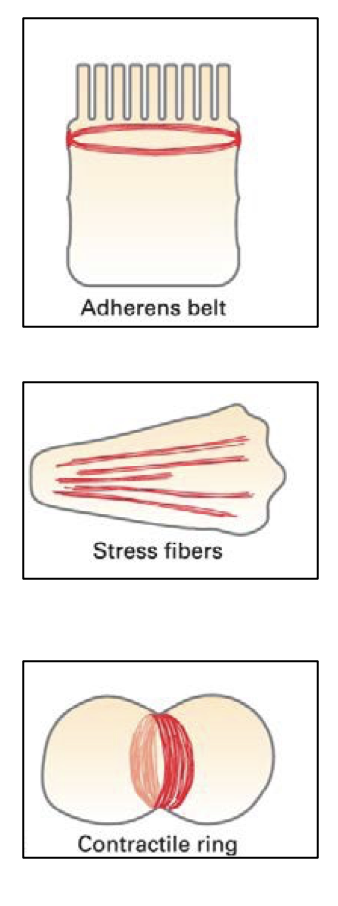
give the 3 main examples of actin and myosin II filament-based contractile bundles in non-muscle cells
Adherens belt – encircles the inner surface of epithelial cells and is important in maintaining the integrity of the epithelium
Stress fibres - along the lower surfaces of cells cultured on artificial (glass or plastic) surfaces, or on extracellular matrices - important in cell adhesion
Contractile ring - transient structure that assembles at equator of a dividing cell, encircling the cell midway between the poles of the mitotic spindle
What is the evidence that myosin II is important in cleavage burrowing in mitosis?
When the gene for the heavy chain of myosin II is deleted, cells are unable toundergo cytokinesis.
Instead, these cells form a multinucleated syncytiumbecause cytokinesis, but not nuclear division, is inhibited