Enzymes
1/53
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
54 Terms
What type of protein are enzymes?
Globular proteins with a tertiary structure
Do enzymes act intracellular or extracellular?
Both!
Intracellular: they are synthesised by living cells and can act within the cell.
Extracellular: they can also be secreted by cells
What is the enzyme’s active site?
A 3D space in the molecule into which a specific substrate molecule can fit and bind.
How does the active site receive its specific shape?
The specific shape is determined by the sequence of amino acids in the polypeptide chain, and the bonds formed between them.
What happens to the active site if the sequence of amino acids in the polypeptide chain changes?
The active site also changes shape and the substrate can no longer bind to the active site as they are no longer complementary.
How is the enzyme-substrate complex formed?
When the substrate and enzyme collide successfully, the substrate fits into and binds to the active site by interactions with R-groups or polar groups of amino acids in the active site.
This forms an enzyme-substrate complex
What factors can affect the ability of the substrate to bind with the R-groups? (2)
temperature
pH
How are new bonds formed in the substrate?
Bonds in the substrate are distorted through the presense of the R-groups, and this increases the chance that they will break e.g. by large changes away from the optimum in temperature or pH.
Breaking of bonds brings new atoms in the substrate closer together and so new bonds can be made.
Activation energy
The minimum energy required for a chemical reaction to take place.
Enzymes as a biological catalyst
When an enzyme-substrate complex is formed, the activation energy is reduced, so the reaction can take place faster.
The enzyme also remains unchanged at the end.
Metabolism
A series of enzyme controlled reactions (anabolic and catabolic)
Two theories of enzyme action
Lock and key hypothesis
Induced fit hypothesis
Lock and key hypothesis (4)
The enzyme is like a lock. The substrate is like a key.
Only one specific key can fit into the lock, and the key must be complementary to the lock. The enzyme’s active site, like a lock has a fixed shape.
As the substrate fits exactly to the enzyme and chemical changes take place and substrate molecules are either broken down or combined to form new products.
The enzyme remains unchanged after the reaction and can be reused.
Anabolism
When multiple substrate molecules are combined by an enzyme to form a single product molecule
Catabolism
The (digestive) enzyme breaks down complex substrate molecules into two or more product molecules.
Induced fit theory hypothesis
The enzyme and substrate are not fully complementary in shape.
As the substrate enters the enzymes active site, forces of attraction between the substrate and R-groups/polar atoms of amino acids in active site are formed.
This causes the shape of the active site to change and stronger bonds are formed with the substrate to fit it better.
Bonds within the substrate are weakened causing the reaction to occur at a lower activation energy.
Example of enzyme using induced fit hypothesis
Lysozyme- digestive enzyme in tears
Effect of pH on enzymes
Amino acids have acid and base groups, so changes in the pH of a solution can change the bonding between amino acids, so altering the secondary/tertiary protein structure.
Change in pH changes the overall charges of R-groups, affecting ability of bonds forming and causing the enzyme to become denatured and inactive.
Inactivation of enzymes (pH)
Caused by small changes in pH, resulting in small, reversible changes in enzyme structure
Deactivation of enzymes (pH)
Caused by large changes in pH away from the optimum that permanently change protein structure
Effect on increase in temperature before reaching optimum (4)
As temperature increases, enzymes and substrate particles gain kinetic energy, so they move faster
causing more frequent successful collisions,
so more enzyme-substrate complexes form,
and the rate of reaction increases until the optimum is reached.
Effect on increase in temperature after the optimum temperature
Increased heat above the optimum temperature gives even more kinetic energy to the particles.
Bonds in the enzyme vibrate and become weaker until they break- weak hydrogen bonds are broken first.
Eventually there is a loss in the secondary and tertiary structure, the 3D shape of the enzymes active site is changed and so the enzyme is no longer complementary and able to catalyse the reaction. The enzyme is denatured.
What can you measure to investigate reactions involving enzymes?
rate of disappearance of substrate
rate of appearance of product
What are the possible independant variables when investing with enzymes?
pH
temperature
concentration of substrate
concentration of enzyme
What are the possible dependant variables when investing with enzymes? (4)
time
volume
mass
absorbance/transmission
How to calculate rate of enzyme-controlled reactions when measuring time
rate = 1/time
e.g. when measuring time taken for colour change at different temperatures.
How to calculate rate of enzyme-controlled reactions when measuring quantity
rate = quantity/time
e.g. when measuring mass of product over time.
Hypothesis of enzyme-controlled reactions
If measuring time taken (for product to form/ reactant to be used), shorter time =
Time at optimum pH/ temperature =
The shorter the time, the higher the rate
Optimum pH/ temperature: The shortest time, the highest the rate.
Experiment improvements
If time/ rate at two values of independant variable are close and optimum is likely to be between these values.
If shortest time/ fastest rate is highest or lowest value of independent variable an you dont know results outside table
IMPROVE: Investigate with more in between values
IMPROVE: extend range of table values
From a graph, how can the initial rate be calculated
Draw a tangent of the graph, going through zero, and work out gradient.
From a graph, how can the rate between two times be calculated
change in y/ change in x
From a graph, how can the rate at a particular point be calculated
Draw a tangent, then work out gradient.
What can be used to maintain a constant pH value
buffer solution
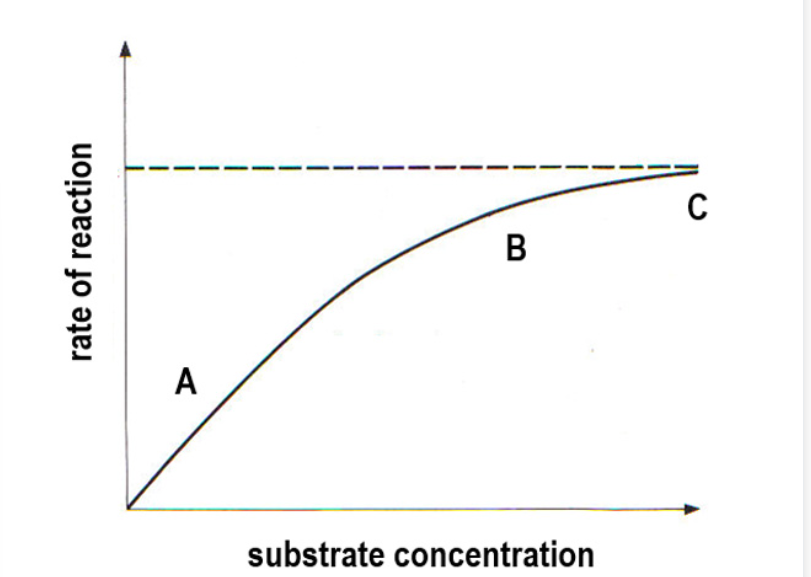
Effect on rate of reaction as you increase substrate concentration
A- At low substrate conc, theres a low number of substrate molecules and not all enzyme’s active sites are occupied. Number of substrate molecules is limiting factor.
B- As substrate concentration increases, there are more enzyme-substrate complexes forming, so increased rate of reaction. More active sites become occupies as substrate concentration increases and there is a smaller increase in rate of reaction.
C- Eventually the maximum rate is reached due to limited amount of active sites, all occupied or saturated. Increasing substrate concentration has no effect and number of enzymes is the limiting factor.
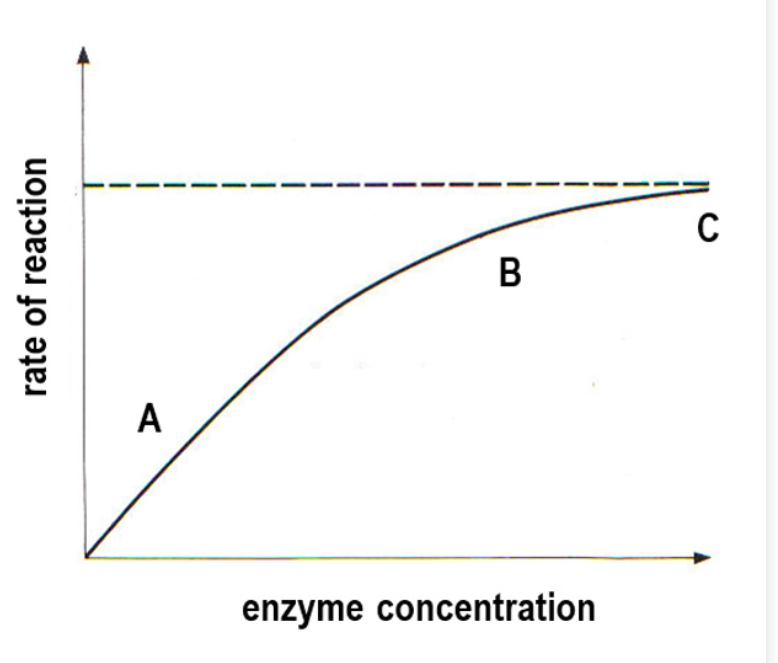
Effect on rate of reaction as you increase enzyme concentration
A- At low enzyme conc, theres a low number of active sites available. Number of enzymes are the limiting factor.
B- As enzyme concentration increases, there are more enzyme-substrate complexes forming, so increased rate of reaction. The substrate concentration is limited so enzyme-substrate complexes are formed at a slower rate, with a small increase in rate of reaction.
C- Eventually the maximum rate is reached due to limited amount of substrate molecules. Increasing enzyme concentration has no effect and number of substrate molecules is the limiting factor.
Enzyme inhibition
When enyzme action is slowed down or stopped by another substance.
When is enzyme inhibition useful?
In cells to control reactions by slowing down or stopping reactions which are no longer needed.
Competitive inhibition
reversible?
when a substance has a close structural resemblance in shape to a substrate molecule and can bind temporarily to the active site instead of the normal substrate.
This blocks the active site so there are fewer enzyme-substrate complexes formed and the rate of reaction is decreased.
This is reversible
What effect does substrate concentration have on competitve inhibition?
It reduces the competition for the active site and maximum rate of reaction can be achieved.
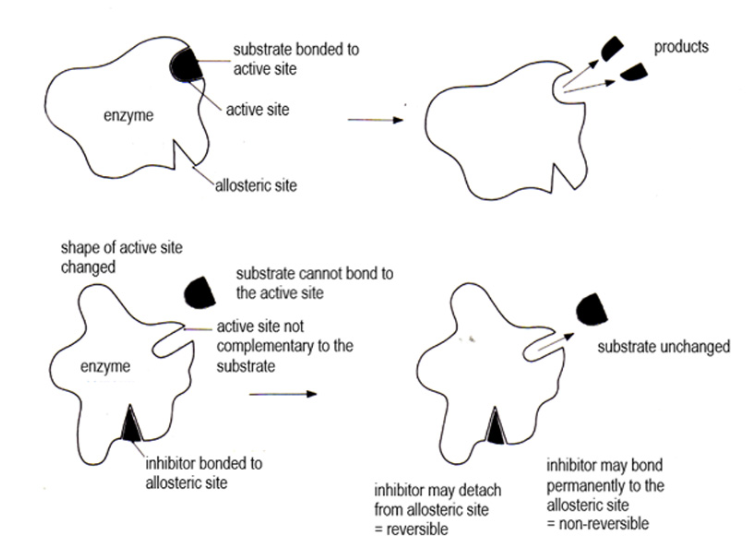
Non-competitive inhibition
name the site
is it reversible?
This is when a substance has no structural resemblance to a substrate molecule, but binds to the enzyme at a point other than the active site, called the allosteric site.
This alters the 3D shape of the active site, so the substrate can no longer bind to the active site, and fewer enzyme-substrate complexes form, and the rate of reaction is decreased
This can be reversible or non-reversible
What effect does substrate concentration have on non-competitve inhibition?
There is no affect, and maximum rate of reaction will not be reached.
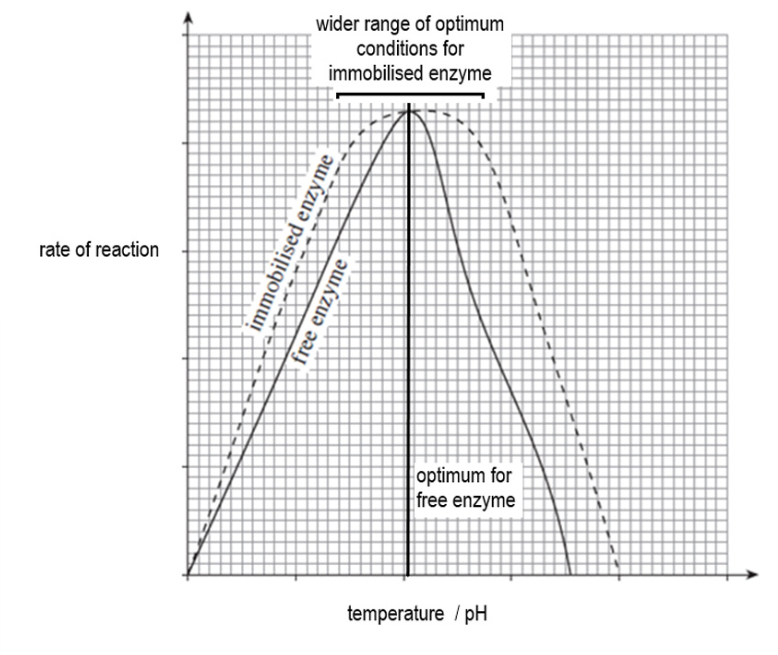
Reason for immobilising enzymes
Immobilising enzymes in an inert substance e.g. alginate, and gel membrane or polyethene, reduces the ability of the polypeptide chain to move and changes in temperature and pH have less effect on the 3D shape of the enzyme.
It stabilises the enzyme, allowing them to have a wider range of optimum pH and temperature.
Benefits of immobilised enzymes in industry (4)
Enzyme can be recovered and reused which reduces costs
It prevents contamination of the product
More flexibility in emperatures can be used
High yields can be reached.
Immobilised enzymes in industry: producing lactose-free milk (3)
This uses immobilised lactase in alginate gel beads
milk is passed over the beads, and enzymes digest the lactose into glucose and galactose
the milk is not contaminated by lactase and beads can be reused
Use of immobilised enzymes in medicine: biosensors or analytical reagents e.g. glucose oxidase electrode
This biosensor is important for diabetics as it can detect glucose levels in the blood.
The enzyme glucose oxidase is immobilised in a gel
the glucose in the blood comes in contact with the enzyme, and a reaction occurs releasing energy, which is converted into electrical impulses.
The more energy released, the higher glucose concentration in the blood.
This can be used to determine an accurate glucose concentration
CORE PRACTICAL: Effect of temperature or pH on enzyme activity
Place a beaker of lipase solution in a water bath of 25 degrees
Place 5cm cubed milk in a test tube and add 5 drops phenolphthalein
Add 7cm cubed sodium carbonate solution and place in 25 degrees water bath to equilibriate.
Add 1cm cubes lipase from beaker in water bath and start stopwatch
Stir the contents of test tube until colour change pink to colourless and record time taken.
Repeat steps for 15, 35, 45, and 55 degrees
For pH testing use buffer solutions with lipase
Sodium carbonate risk assessment
Irritant, may enter eye, where saftey goggles
Phenolphthalein risk assessment
Contains ethanol which is flammable, using bunsen burner for water bath could ignite ethanol, do not use ethanol near bunsen burner
CORE PRACTICAL: Effect of temperature or pH on enzyme activity → EXPLANATION
In this investigation, an alkaline solution or milk, lipase and phenolphthalein will change from pink to colourless as the fat in milk is broken down into fatty acids and glycerol, so reducing the pH.
The time taken for this reaction to occur is affected by temperature.
CORE PRACTICAL: effect of substrate concentration on enzyme activity
Grind a 2cm piece of potato cylinder with 5cm cubed of distilled water to make a smooth paste containing enzyme
Place 10cm cubed of H2O2 in a test tube.
Using forceps, dip a filter paper disc into the enzyme suspension, tap of the excess
Drop the filter paper disc into the hydrogen peroxide solution and measure time taken from it striking the surface to sink, then float up to the surface again.
change conc. of H2O2
Hydrogen peroxide risk assessment
Irritant, may splash into eyes, wear safety goggles
CORE PRACTICAL: effect of substrate concentration on enzyme activity → EXPLANATION
Why are potatoes used and what is the reaction taking place?
Catalase is found inhigh concentations in raw potatoes. It is an enzyme that breaks down hydrogen peroxide toxin.
2H2O2 → 2H2O +O2
CORE PRACTICAL: effect of an inhibitor on enzyme catalysed reaction
Measure 5cm cubes yeast suspension using a 5cm cubes syringe and tranfer yeast into a beaker
Add 1cm cubed distilled water by syringe to yeast in beaker and mix well with glass rod
Use a measuring cylinder and add 10cm cubed of 10 vol H2O2 in a test tube
Use forceps and dip a disc of filter paper into yeast suspension and then drop disc into H2O2. Record time taken for disc to sink then rise again.
Repeat using a new paper disc and the same H2O2 solution to gain another reading
Repeat steps for 2 vol H2O2
measure another 5cm cubed yeast suspension and place in a separate 100cm cubed beaker
Add 1cm cubed CuSO4 to yeast suspension and mix well
Repeat steps usuing yeast suspension and CuSO4 mixture.
CORE PRACTICAL: effect of an inhibitor on enzyme catalysed reaction → EXPLANATION
CuSO4 is used as a pesticide and is toxic if ingested. In humans, copper sulfate interacts with iron-containing haem group in haemoglobin and reduced its ability to transport oxygen.
Catalase is an enzyme that contains iron and is inhibited by copper sulfate. It is found in yeast and breaks down hydrogen peroxide into water and oxygen.