Intro to Biotech Exam IV
1/114
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
115 Terms
The immune system is an important component in
noninfectious diseases! It can prevent/promote cancer, and is in involved in responses towards organ transplants/autoimmune diseases.
The 1st line of defense needs to be circumvented before the….
…immune system is activated.
What are the different aspects of the immune system?
Mechanical (skin, epithelial cell junctions)
Chemical (fatty acids on skin, acid/enzymes in gut)
Microbiological (flora on gut/skin)
How are immune cells derived, overall (THINK ALL IMMUNE CELLS ARE DERIVED FROM THIS)?
From hematopoietic stem cells in the bone marrow.
What immune cells are derived from tissues?
Mast cells, immature dendrites, macrophages.
What immune cells are derived from blood?
Neutrophils
What immune cells are derived from the lymph progenitor line?
B cells/T cells
Innate (quick response) =
most immune cells
Adaptive (slow) response) =
B/T cells
In both innate and adaptive response, there are…
T/natural killer cells.
What do B-cells create?
Antibodies!
What types of T cells are there?
CD4 and CD8.
What cells play a vital role in adaptive response?
Dendritic (although they’re also usually innate) cells.
What are the “first responder” innate response cells?
Macrophages
Describe adaptive immunity with “yes'“ terms:
Yes memory, yes specificity, BUT doesn’t things broadly.
Days - weeks.
Describe innate response with “no” terms":
No memory, no specificity, but DOES HAVE a broader perspective.
Minutes - hours.
Overall, 0-96 hours of innate immunity.
Describe the process of infection establishment
After 0-96 hours of innate immunity, infection establishes itself by crossing over the threshold antigen level to activate the adaptive immune response.
The pathogen is cleared, immunological memory is formed (vaccines also introduce immunological memory).
If you have NO adaptive immunity, you have NO way to clear infection.
Physical layer breach → bacterial entry, macrophages activated, bring in additional innate cells such as cytokines.
What do macrophages do?
Possess receptors that allows them to detect microbes/foreign agents.
They trigger innate immune responses and secrete inflammatory mediators when they detect microbes to amplify the inflammatory response.
Inflammatory mediators promote…
…vascular changes, they increase dilation and permeability. Causes vasculature to become “leaky” → redness/pains.
Immune cells need to be able to get to….
…the infection, this is why innate immune cells affect vasculature. The creation of “gaps” to promote access to vasculature.
What do cytokines (particularly IL-1, TNF-α) do?
They help endothelial cells guide other immune cells into tissue + stimulate endothelial cells to secrete adhesion molecules to slow blood flow.
Secreted by macrophages.
What do leukocytes do?
They’re blood neutrophils that into the tissue, then clear everything up and cause pain/redness.
If infection is not cleared within 6 hours, what kicks in?
Adaptive immune response.
Complement system →
A facet of innate immunity of factors produced by the liver that increases inflammation by binding/killing microbes
What do NK (natural killer) cells do?
They target normal but infected cells, they’re a facet of innate immunity and of the complement system.
What does the lymphatic system do?
It picks up stuff, drains tissue fluid from the blood, not pressurized.
Explain transport via lymphatic vessels.
The cells head out to reach secondary lymphoid tissue to be activated (primarily, the lymph node).
Lymphatic fluid enters the lymph node and cycles through the blood until death or activation is cued, then they leave the lymph node into circulation and migrate to the specific site that they’re needed at.
What is clonal expansion( part of the adaptive immune response)?
Remember that there are multiple flavors of B/T cells. Effective adaptive immune response → once cell is activated, it gets cloned → clonal expansion, clonal army that goes after a specific target.
Huge repertoire initially, then activate clonal army in lymph node.
What do dendritic cells do?
They gobble up material and travel to the lymph node, during travel they change so that they can interact with other immune cells, particularly T cells.
There are two subsets of T cells, what are they?
CD4: helper (MHC Class II) T cells that activate CD8 T cells and are responsible for antibody response.
CD8: These are cytotoxic T cells that are MHC Class I and kill other cells.
Both are in the lymph node.
What is the dendritic-T cell interaction?
KEY FOR T CELL RESPONSE!
Particularly interactions with CD4 T cells. Numerous flavors, different immune reaction ability of helper T cells to trigger CD8 T cell response or provide antibodies.
What are the first lines of defense?
Before the cells of the immune system are engaged in fighting infection, you have to get through commensal microorganisms (microbiota), physical and mechanical barriers, and antimicrobial substances like enzymes and peptides in body secretions (produced by epithelial cells and phagocytes) like tears/mucus/saliva
Innate immunity cells and mediators act when…
Physical and chemical barriers are overcome or evaded (wounds/burns/skin and mucosal ulcers).
Innate immunity acts within minutes - days since the danger is detected and often prevents the developments of signs/symptoms of disease.
Time is Key!
Innate immunity (immediate, 0-4 hrs)
Early induced innate response (early, 4-96 hours)
Adaptive immune response (late, more than 96 hours).
What are the phases of the infectious process?
1.) Pathogens adhere to epithelium.
2.) Epithelium infection/penetration.
3.) Tissue infection
4.) Adaptive immune response kicks in.
Preface to the vaccine era - what is pre-germ and post germ theory?
•Edward Jenner (Late 1700s)
–Noted that milkmaids infected with cowpox seemed to be protected against smallpox
•Farm lore: “I shall never have smallpox for I have had cowpox. I shall never have an ugly pockmarked face.”
–Inoculated 8 year old boy with puss from milkmaid’s hand
•6 weeks later à infected child with smallpox
Inoculation protocol spread throughout Europe but was delayed worldwide until 1800s
WHO smallpox eradication campaign
(1960s & 1970s):
Last known natural infection, Somalia 1977
(Late 1800s)
•Pasteur and Koch
–Microorganisms cause infectious disease
•With improved knowledge and development of techniques, could begin to devise vaccines
•
•Onslaught of vaccine development for humans
–Rabies (1885)
–Typhoid (1896)
–Cholera (1896)
Plague (1897)
All these from 19th century only.
What is the overview of “Jennerian inoculation”?
Cowpox + smallpox viruses share some surface antigens.
Immunization with cowpox induces antibodies against cowpox surface antigens.
Cowpox antibodies bind to and neutralize the smallpox virus.
Method not possible for other major human viral pathogens → no known mild/safe counterpart.
What is vaccination? What are the two types of vaccination methods?
A deliberate method to manipulate the immune system and provide protective immunity against disease.
There is prophylactic (disease prevention) and therapeutic (treatment of existing disease)
What are the major-types of FDA approved vaccines?
Live attenuated (live pathogen that is mutated, no longer pathogenic to humans)
Killed/inactivated (pathogen that can’t replicate due to treatment with a fixative such as formalin/DNA-damaging irradiation/heat).
Subunit (immunogenic components of a pathogen’s surface, components such as proteins and polysaccharides)
Toxoid (purified toxins produced by pathogens that are inactivated by formalin treatment but still antigenic)
Conjugate (conjugation of an antigen, e.g. a polysaccharide to an immunogenic protein, e.g. a toxoid to provide T cell help and stimulate polysaccharide-specific B cells to make antibodies. Needed for children less than 18 months and elderly that make ineffective T-independent responses)
Also, mRNA vaccines!
What type of vaccines are the measles, mumps, and rubella vaccines?
Live-attenuated
What type of vaccine is the Salk polio virus vaccine?
Killed/inactivated.
What type of vaccine is the HBsAg vaccine?
Subunit vaccine.
What type of vaccine are the diphtheria and tetanus vaccines?
Toxoid vaccine.
What type of vaccines are the H. influenza and pneumococcus vaccines?
Conjugate vaccines
How do killed/subunit/toxoid vaccines work?
Antibodies primarily promote host protection.
Dendritic cells migrate via afferent lymphatics to regional lymph nodes, the mature dendritic cell is in the deep cortex.
Activated B cell → somatic hypermutation of immunoglobulin V-regions in rapidly proliferating germinal center B cells
Either of the two happens:
Germinal center B cell with mutated low-affinity surface immunoglobulin, B-cell receptor is not cross-linked and B cell cannot present antigen to T cell, B cell dies by apoptosis.
Germinal center B cell with mutated high-affinity surface immunoglobulin, T cell help and B cell receptor cross linking sustain B-cell proliferation and maturation.
→When the vaccine is administered, the body recognizes the foreign material (killed germ, subunit, or toxin) as an invader.
The immune system then produces antibodies and other immune cells to fight off the "invader".
These antibodies and immune cells "remember" the pathogen, so if the real pathogen enters the body later, the immune system can quickly recognize and destroy it, preventing or reducing the severity of the disease.
How does a conjugate vaccine work? T-independent B cell activation?
Conjugate vaccine: The B cell binds bacterial polysaccharide epitope linked to tetanus toxoid protein, antigen is internalized and processed, peptides from protein component are presented to the T cell resulting in T-dependent activation, then activated B cell produces antibody against polysaccharide antigen on the surface of the bacterium.
T-independent B cell activation: This process tends not to occur very well in younger/older patients, also the process does not usually give a very “good” IgG antibody response or memory B cells, hence the need for conjugate vaccines for all patients.
How do you make a better antibody molecule?
Repeated immunizations will usually give…
class switching → affinity maturation,
Helper T cell cues.
Development of high affinity B cells, competition for antigen binding: stimulation + clonal expansion.
Primary IgM → class switch to IgG → somatic hypermutation to a high affinity IgG.
What are major antibody-based mechanisms for host protection?
1.) Bacterial toxins to cell with receptors for toxin → neutralization → ingestion by macrophage
2.) Bacteria in extracellular space come in to contact with macrophages → opsonization → ingestion by macrophage
3.) Bacteria in plasma → complement activation → lysis and ingestion.
What are the antibody classes?
IgM - complement, opsonization
IgA - protects mucosal surfaces
IgG - general systemic protector
IgE - parasites, allergies
Antibody class distribution
Blood - IgM, IgG
Extracellular fluid - IgG, monomeric IgA
Secretions across epithelia - dimeric IgA
Below epithelial surfaces - IgE
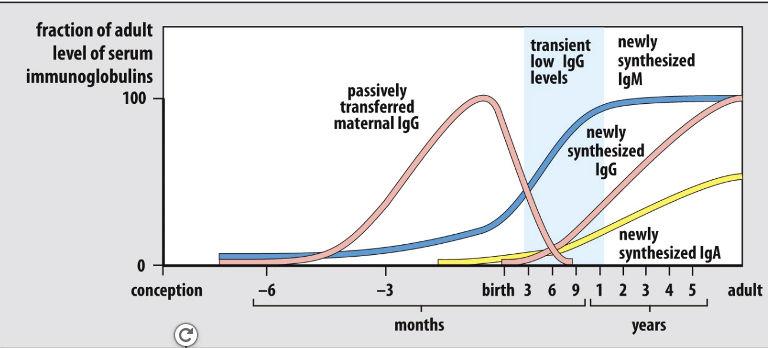
Fetus receives antibody passively, newborn baby receives IgA and IgG through breast milk.
Antibody from breastmilk is an important component to providing a growing child protection
against pathogens. However, these antibodies can interfere with certain live attenuated vaccines
(i.e., a concept known as maternal antibody interference).
Antibody profiles during human development
Advantages and disadvantages of killed/inactivated vaccines
Advantages:
Safe - no risk of pathogen mutating and reverting to virulent state |
Stable - usually require no refrigeration |
Less affected by pre-existing antibodies |
Disadvantages:
Stimulate relatively weak response |
Repeated administration necessary for effective immunity |
Antigens may not be preserved during the inactivation process |
Poor inducers of CD8+ T cell and mucosalimmunity |
Live attenuated vaccine results in….
CD8+ T cells that promote host protection.
A naive T cells sees an antigen, the vax. interacts with the dendritic cell which interacts with the T cell, the effector T cells differentiate and secrete cytokines/express cytokine receptors which leads to cytotoxic T cells [CD8 cells - peptide + MHC Class I] that kills virus-infected cells.
Development of Live-Attenuated Vaccine Strains
Tissue culture passage (traditional approach) - growth of agent in non-human cell lines results in mutations that render the pathogen less infectious to humans.
Pathogenic virus is isolated from a patient and grown in human cultured cells, cultured virus is used to infect monkey cells, virus acquires many mutations that allow it to grow well in monkey cells, virus no longer grows well in human cells (it is attenuated) and can be used as a vaccine.
Recombinant DNA technology: Create avirulent (nonpathogenic) virus or bacterial pathogen for a vaccine:
Mutate or delete a gene necessary for virulence but not growth or immunogenicity
Normally disrupt many virulence genes so that the pathogen cannot easily revert to the wild type.
As an example: Approach being attempted for malaria
Isolate pathogenic virus, isolate virulence gene, then you can either mutate virulence gene or delete virulence gene, resulting virus is viable and immunogenic, but avirulent. Can be used as a vaccine.
Advantages and disadvantages of live-attenuated vaccines
Disadvantages |
Can revert to virulent strain by mutation |
Can cause serious illness in immunosuppressed individuals |
Stringent storage instructions to maintain vaccine efficacy and safety |
Cannot vaccinate in early infancy due to maternal antibody interference |
May contain adventitious agents (contaminating pathogens) |
Advantages |
Preserve immunogenicity of virulent agent such as conformational antigens |
Mimic natural infection more effectively |
Stimulate multiple components ofthe immune system such as |
Vaccine considerations
•Adjuvant (Enhancing substance)
–Potentiates antigen presenting cell activity
•Convert soluble protein into particulate matter
•Elicit controlled inflammation to induce protective immunity
–FDA approved for human use
•Aluminum salts
–Examples: DTaP, pneumococcal conjugate, and hepatitis B vaccines
•AS03 (oil in water emulsion)
–D,L-alpha-tocopherol (vitamin E) + squalene + polysorbate 80
–Example: H5N1 influenza vaccine
•AS04
–Aluminum hydroxide + monophosphoryl lipid A
–Example: Cervarix (HPV Types 16 & 18)
•Frequency
–Ensure immunological memory
•Route of administration
–Injection, inhalation, ingestion
•Induce appropriate immune response
•Mimic natural route of infection?
Herd immunity
Community health even though not everyone is immunized
However, a certain level of vaccine
coverage must be achieved for each
pathogen to achieve herd immunity
Estimated Coverage Thresholds
Diphtheria 85%
Pertussis 92-94%
Polio 80-86%
Measles 83-94%
Mumps 75-86%
Rubella 83-85%
How do we know a vaccine safely works?
•Clinical trials
–Phase I-III trials à FDA approval
•Post-licensing safety monitoring
–National program for reporting adverse events
•Vaccine Adverse Event Reporting System (VAERS)
•Population-level data
Annual cases versus historical data
Efficacy of large-scale vaccination
Even though cases aren’t a problem in the U.S., vaccine campaigns must continue due to worldwide incidence
Characteristics of a good vaccine
Safety | -Must not cause illness or death -Few side effects |
Elicits strong protective immune response | -Protect against illness resulting from exposure to disease-causing agent -Generate robust immunity (e.g., neutralizing antibodies, T helper and/or cytotoxic T cell responses) |
Long-lasting protection | -Protect against illness for expected duration of exposure |
Cost effectiveness | -Wide global applications in regions most at need |
Stability | -Ideal vaccines are those that retain long-term efficacy at room temperature *Many vaccines will be needed in countries without a reliable cold chain |
Ease of administration | -Efficacy requires proper administration by health professionals |
Examples of targetable diseases where effective vaccines are unavailable
Disease | Estimated annual |
Malaria | 627,000 |
Tuberculosis | 1.6 million |
HIV/AIDS | 650,000 |
Cancer | 10 million |
What are some common challenges in vaccine development?
•Immunogenicity
–Immune response does not elicit protection (e.g., HIV)
–Pathogen remains invisible to the immune system (e.g., HSV-2)
•Lack of immune system understanding
–Generating effective T cell memory
–Vaccine efficacy in developing countries
•Lack of pathogen understanding
–During natural protection: How the immune system interacts?
•High mutation rate of pathogen
-One disease may be caused by several individual pathogens or subtypes
•Industry interest
Why can generating a protective CD8+ T cell response from vaccines be challenging?
It requires the delivery of antigens to the cytosol in order to allow for processing and presentation by MHC Class I + activation of inflammatory responses that facilitate the proper co-stimulation of antigen-specific CD8+ T cells.
Some types of pathogens (like intracellular bacteria, viruses) require CD8+ T cells for protection.
Vaccine safety
•All approved vaccines have a strong safety record
–Major current fears:
•Autism-spectrum disorders
–1998 Lancet paper that first reported the link à
Found fraudulent and withdrawn in 2010
–Institute of Medicine (IOM) comprehensive review (2004) à
No causal relationship between certain vaccine types and autism
•
•“Too many vaccines” – Overburden the immune system
–Number of vaccine antigens delivered to children by
2 years of age
»Late 1990s: Several 1000s
»2013: 315
•
•Fear and hype contribute to reduced childhood vaccination and increased disease incidence
First lines of defense
Commensal microorganisms (microbiota)
Physical, mechanical barriers - skin, mucosal surfaces on the gut/respiratory tract/eyes.
Antimicrobial substances (enzymes and peptides) in body secretion, produced by epithelial cells and phagocytes, in tears/mucus/saliva.
Mechanical - flow of air or fluid, skin/gut.
Lungs - movement of mucus by cilia.
Eyes/nose/oral cavity - tears, nasal cilia.
Innate immunity cells and mediators act when…
Physical and chemical barriers are overcome/evaded (wounds, burns, skin and mucosal ulcers, physical barrier’s integrity is compromised).
Innate immunity acts within minutes - days since the danger is detected and often prevents the development of signs and symptoms of disease.
Definition + characteristics of innate immunity:
Oldest type of immunity, always and immediately available.
Responds to physical or chemical injury or tissue damage.
Occurs rapidly on exposure to infection or injury.
Does not generate specific response and immunological memory, alarms adaptive immunity.
Serves as an effector for adaptive immune responses.
Time is key!
Innate immunity (immediate, 0-4 hours): infection, recognition by performed/nonspecific and broadly specific effectors, removal of infectious agents.
Early induced innate response (early, 4-96 hours): infection, recruitment of effector cells, recognition of PAMPS/activation of effector cells + inflammation, removal of infectious agent.
Adaptive immune response (late, >96 hours): infection, transport of antigen to lymphoid organs, recognition by naive B and T cells, clonal expansion and differentiation to effector cells, removal of infectious agent.
Phases of infectious process.
1.) The pathogens adhere to epithelium, tissue macrophage + dendritic cells are waiting.
Local infection + penetration of epithelium.
Local infection of tissues, lymph node becomes involved.
Adaptive immunity comes into play and brings out antibody dependent cytotoxicity + T and B cells.
What are the components of innate immunity?
Cells (think macrophages, dendritic cells, granulocytes)
Soluble mediators (think antimicrobial peptides such as blood/EC fluids/epithelial secretions, lysozymes in tears/saliva/human milk/mucus, complement system, cytokines, chemokine, arachidonic acid cascade products such as leukotrienes/prostaglandins [EMPHASIS], coagulation factors, kin and bradykinns)
There are three types of macrophages:
Ubiquitous resident macrophages: always present, first responders to pathogens.
Embryonic origin/hematopoietic - they migrate to tissues prior to hematopoiesis, and self-renew.
•LANGERHANS CELLS (SKIN)
•MICROGLIA (BRAIN)
•ALVEOLAR MACROPHAGES (LUNG)
•KUPFFER CELLS (LIVER)
Peritoneal/pleural macrophages: inflammatory, they originate from monocytes that migrated to tissues.
What do macrophages do?
They’re receptors recognizing danger, engage in phagocytosis (pathogen neutralization/present antigens), produce + secrete inflammatory mediators, clear dead cells and cell debris.
What are granulocytes?
Innate immune response cells that go to the site of infection and kill bacteria/produce inflammatory mediators.
Phagocytosis and pathogen neutralization is performed via neutrophils.
Or they can be involved in defense against parasites, involved in allergies via eosinophils/basophils.
How do neutrophils kill microbes?
Oxygen active species kill bacteria!
Neutrophils engulf + kill microbes to which they bind, bacterial fMet-Leu-Phe peptides activate Rac2, and bacteria are taken up into phagosomes.
Phagosomes fuse with primary/secondary granules. Rac2 induces assembly of a functional NADPH oxidase in the phagolysosome membrane, leading to generation of O2-, acidification as a result of ion influx releases granule proteases from granule matrix.
What are mast cells?
First responders that secrete vasoactive and inflammatory mediators.
Think:
•TRYPTASE
•SEROTONIN
•HISTAMINE
•HEPARIN
•THROMBOXANE
•PROSTAGLANDINS
•LEUKOTRIENS
What are the two types of dendritic cells?
•Conventional: perform antigen engulfment, process/present in MHC II. Activate T cells.
Plasmocytoid: involved in antiviral immunity.
Dendritic cells need to mature to….
…stimulate T cells.
When an immature dendritic cell comes into contact with a pathogen in the blood vessel, antigen uptake is performed via endocytosis/phagocytosis/macropinocytosis, followed by the immature dendritic cell traveling to the lymph node and becoming mature, now there is antigen presentation and T-cell activation via the dendritic cell.
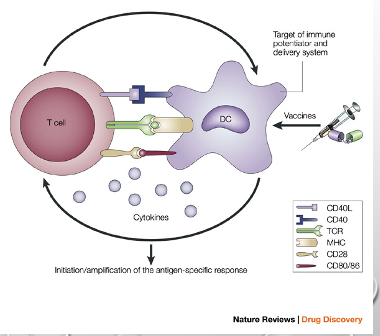
Dendritic cells activate T cells in lymph nodes [IMPORTANT], what are the 3 signals required to activate the T cells?
Peptide MHC II complex, costimulatory molecules (CD80 + CD86), cytokines.
Think TCR, think TCR MHC II.
What are natural killer cells?
They kill infected/tumor cells and intracellular pathogens.
What are endothelial cells?
They’re in the vasculature!
Active contributors to inflammatory response, they facilitate extravasation of leukocytes in inflammation sites.
How do macrophages and other components of innate immunity recognize danger?
Innate immunity recognizes btw: self, self-modified, and non-self.
Non-specific recognition, no immunological memory.
Activated when encounters non-self/self-modified, it recognizes structures that are typical for pathogens/modified host cells.
•PATHOGEN ASSOCIATED MOLECULAR PATTERNS (PAMPs)
•DANGERS ASSOCIATED MOLECULAR PATTERNS (DAMPs)
To recognize PAMPs/DAMPs innate immunity utilizes pattern recognition receptors (can be membrane bound/secreted, encoded in the germline, some of them serve only as facilitators of phagocytosis whereas others initiate intracellular signaling)
What are the different receptors on phagocytes?
TCRs (toll like receptors) → plasma membrane or nucleus.
Activate TCR → activate NF-kB transcription factor [VERY IMPORTANT FOR EXAM] → this TF attaches to DNA and activates gene transcription which is important for inflammatory response via encoding pro-inflammatory cytokines such as TNF-α, IL-1B, and IL-6.
Complement/intracellular receptors (facilitate interaction with different pathogens):
•CR1 – C3b/C4bi : promote C3/4b decay, when C5a is present they stimulate phagocytosis.
CR3 and CR4 – iC3b - stimulate phagocytosis
What is the complement system?
Plasma + membrane bound protein assembly (around 50 total) that contributes to antimicrobial defense + inflammation and bridges the gap between innate and adaptive immunity.
ENHANCES ANTIBODY MEDIATED KILLING OF PATHOGENS
When complement regulatory proteins prevent complement activation on host cell surface, remember that the complement cascade is controlled by inhibitory molecules that are expressed on…..
ON CELLS.
The cells are protected!
What are the different pathways of immune response?
Alternative pathway - always on
Classical pathway - activated by immune complexes.
Lectin pathway - think bacteria.
In every pathway, C3b is deposited and is cleaved into C3a, which activates inflammation, C3b is involved in phagocytosis, also there’s a formation of membrane attack complex (MAC) which causes lysis of microbe [think TCC]
What are cytokines and chemokines?
Proteins made by cells that act on target cells via receptors or in an autocrine/parachrine/endocrine manner.
Chemokines are cytokines that are chemoattractants.
Both can be inflammatory mediators.
What are the cytokines and chemokine released from macrophages/dendritic cells during the innate immune response?
IL-1 B, IL-6, IL-12, TNF (think inflammation)
CXCL8 (IL-8) - on humans only
Inflammation
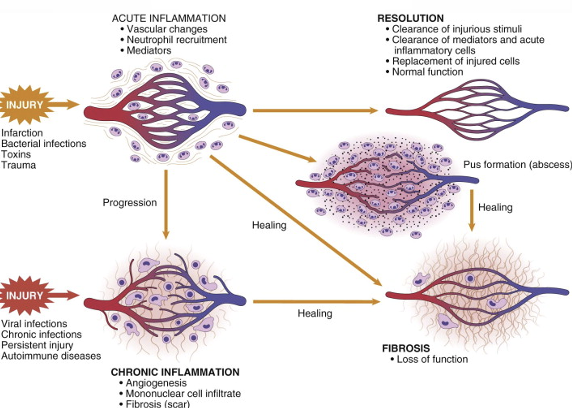
If innate immunity can’t decrease danger instantly, it creates an inflammatory response that enhances it [innate immunity].
Inflammation is basically the interactions between soluble factors and cells in response to trauma/injury/disease.
Normally leads to healing, but if targeted destruction/assisted repair are not properly phased, inflammation can lead to persistent tissue damage by leukocytes, lymphocytes, or collagen.
Main feature of inflammation: neutrophil and macrophage recruitment to site of infection or injury.
This describes acute! When acute becomes chronic, this causes several diseases.
•SIGNS OF INFLAMMATION
•COLOR - HEAT
•DOLOR - PAIN
•RUBOR – REDNESS
•TUMOR - SWELLING
FUNCTIOLAESA– LOSS OF FUNCTION
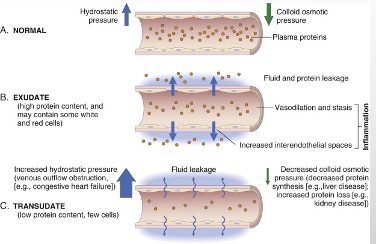
Reactions of blood vessels in acute inflammation [spent a lot of time on this]
•CHANGES IN THE FLOW OF BLOOD AND THE PERMEABILITY OF VESSELS, BOTH DESIGNED TO MAXIMIZE THE MOVEMENT OF PLASMA PROTEINS AND LEUKOCYTES OUT OF THE CIRCULATION AND INTO THE SITE OF INFECTION OR INJURY
What is vasodilation induced by ?
Mediators, especially histamine, on vascular smooth muscle.
Very early sign of acute inflammation.
First arterioles → opens new capillary beds on the area, resulting in increased blood flow causing heat/redness aka erythema at inflammation site.
Increased permeability of the microvasculature
•MECHANISMS
Endothelial cell contraction ( an immediate transient response around 15-30 minutes in), followed by endothelial injury (necrosis + detachment), followed by increased transport of fluids and proteins [transcytosis] through the endothelial cell.
•CAUSES
1.HISTAMINE, BRADYKININ, LEUKOTRIENES & OTHERS
2.MICROBES, MICROBIAL TOXINS & NEUTROPHILS
●VEGF
What does stasis (slower blood flow) do?
Less fluid + increased vessel diameter leads to slower blood flow, more red cells in small vessels, and more viscous blood, resulting in engorgement of small vessels with slowly moving red cells, aka vascular congestion and localized redness of involved tissue.
During stasis, blood leukocytes (neutrophils, mainly) accumulate on the vascular endothelium, during this the endothelial cells are activated by mediators produced at sites of infection + tissue damage and express more adhesion molecules.
Leukocytes adhere to the endothelium and migrate through the vascular wall into interstitial tissue.
What is extravasation?
Fluid leakage due to lymphocytes getting closer to the endothelial cells and starting to roll → attach (integral activation by chemokines), stable adhesion, migration through endothelium.
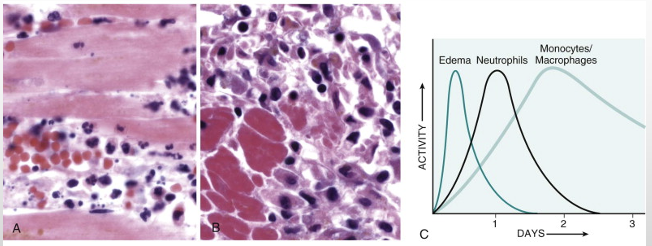
First neutrophils then macrophages:
Myocardial muscle shown.
Second photo - no nuclei.
Inflammation is a response to tissue necrosis.
Outcomes of inflammation
What are challenges in vaccine development?
Immunogenicity (immune response does not elicit protection, e.g. HIV or pathogen remains invisible to the immune system, e.g. HSV-2 which hides from the immune system).
Understanding of pathogen (how does the immune system interact)?
Lack of immune system understanding (how do you generate effective T cell memory? Vaccine efficacy in developing countries).
High mutation rate of pathogen, one disease may because by several individual pathogens or subtypes, industry interest.
What is the immune system’s role in preventing/controlling cancer?
Immunosurveillance!
Viable tumor cells that are chemically induced are irradiated, which killed the tumor cells.
They made a killed vaccine that immunized mice prophylactically that prevented tumors in mice.
Novel antigenic targets can arise in tumors and be recognized by the immune system.
What’s the concept of immunoediting -
Two groups of mice: wild type and immunocompromised.
When they introduced tumors in animals that are immunocompromised, two types of tumors developed - strongly immunogenic tumors [should’ve been seen by the immune system] and weakly immunogenic tumors [not seen by immune system]
In wild type mice, only weakly immunogenic tumors were seen [that the immune system can’t see].
Explain immunosurveillance
Immunosuppressed - cancer incidents rate increases, implying that the immune system helps prevent the formation of cancer, this is the concept of immunosurveillance.
If you take someone that has a solid tumor, you excise that tumor and look at the types of cells in it, you can stratify patients based on 2 characteristics: lots of CD8 T cells or not.
Prognosis is heavily reliant in how many CD8 T cells they have [more in tumors = good].
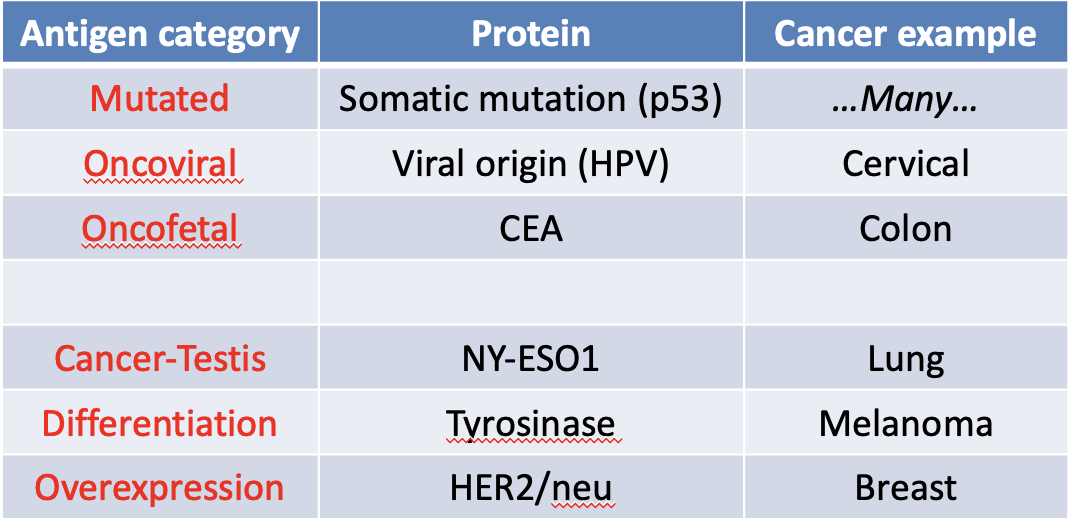
How can anti-tumor immunity proceed despite host tolerance mechanisms?
Immunotherapy strategies are largely based on these tumor specific/associated antigen categories.