Introduction to Chemistry
1/13
Earn XP
Description and Tags
A set of flashcards summarizing key terms and concepts related to chemistry, including definitions and explanations.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms

Chemistry
The study of what matter is made of and how substances change.
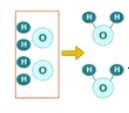
Reactant
A starting material in a chemical reaction.
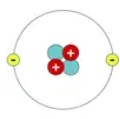
Atom
The smallest building block of matter; everything is made of atoms.
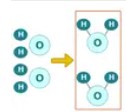
Product
A substance made at the end of a chemical reaction.
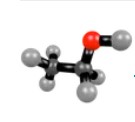
Molecule
Two or more atoms joined together.
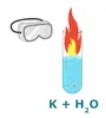
Chemical Reaction
A process where substances change into new substances.

Element
A pure substance made of only one kind of atom (like oxygen or carbon).
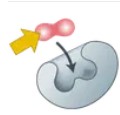
Enzyme
A protein that speeds up chemical reactions in living things.
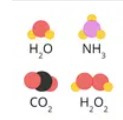
Compound
A substance made of two or more different elements chemically joined.
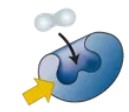
Substrate
The specific molecule an enzyme works on.

Catalyze
To speed up a chemical reaction.
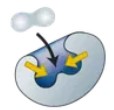
Active Site
The part of an enzyme where the substrate fits and the reaction happens.
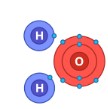
Covalent Bond
A chemical bond where atoms share electrons.
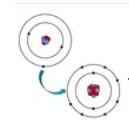
Ionic Bond
A chemical bond where one atom gives electrons to another.