SCH4U - U1a Atomic Structure
1/112
Earn XP
Description and Tags
practice test + *2023 test questions
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
113 Terms
Which of the following scientists proposed that the atom cannot be divided or destroyed?
a. | Thompson | c. | Rutherford | e. | Dalton | |
b. | Bohr | d. | Einstein |
e. | Dalton |
2. Which observation led J.J. Thompson to conclude that negative particles existed within the atom?
a. | deflection of alpha particles |
b. | atomic absorption spectra |
c. | atomic emission spectra |
d. | the deflection of cathode rays by an electric field |
e. | absorption of beta particles |
d. | the deflection of cathode rays by an electric field |
3. Rutherford, through experimentation, first identified the presence of _________ in the atom.
a. | Neutrons |
b. | Photons |
c. | Electrons traveling in orbits |
d. | Answer a & b are correct |
e. | all of the above are correct |
c. | Electrons traveling in orbits |
4. Which of the following are examples of electromagnetic radiation?
a. | Visible light |
b. | X rays |
c. | Microwaves |
d. | Answers a & b are correct |
e. | Answers a, b & c are correct |
e. | Answers a, b & c are correct |
a. | Visible light |
b. | X rays |
c. | Microwaves |
5. Of the following types of electromagnetic radiation, which has the greatest frequency?
a. | Radio waves | c. | uv rays | e. | Infrared rays |
b. | X rays | d. | Microwaves |
b. | X rays |
Radio waves → Microwaves → Infrared rays → Visible light → Ultraviolet (UV) rays → X-rays → Gamma rays
6. Of the following types of electromagnetic radiation, which has the shortest wavelength?
a. | Gamma rays | c. | uv rays | e. | Microwaves |
b. | Infrared rays | d. | X rays |
a. | Gamma rays |
7. Given the following types of electromagnetic radiation, __________ radiation has the longest wavelength and __________ radiation has the highest frequency.
microwaves ultraviolet visible
a. | microwaves, visible | d. | visible, microwaves | |
b. | microwaves, ultraviolet | e. | ultraviolet, microwaves | |
c. | visible, ultraviolet | |||
b. | microwaves, ultraviolet |
8. Which colour of visible light carry the highest energy?
a. Blue | b. Green | c. Violet | d. Red | e. Yellow |
c. Violet
9. As wavelength ___________ and frequency __________, the energy carried as electromagnetic radiation increases.
a. Decreases, decreases | c. Decreases, increases |
b. Increases, Decreases | d. Increases, increases |
c. Decreases, increases
10. Which of the following best describes a photon?
a. | a radioactive wave d. a sound wave |
b. | a gamma ray e. a continuous spectrum |
c. | a discrete bundle of energy |
c. a discrete bundle of energy
11. Of the following contributions to the quantum theory of the atom, Planck proposed
a. | a relationship between mass and energy |
b. | the uncertainty principle |
c. | matter exhibits wave-particle duality |
d. | light exhibits wave-particle duality |
e. | none of the above are correct |
a. | a relationship between mass and energy |
12. The energy of a photon of light is __________ proportional to its wavelength and __________ proportional to its frequency.
a. | inversely, inversely |
b. | directly, directly |
c. | directly, inversely |
d. | inversely, directly |
d. | inversely, directly |
13. Bohr believed that electrons can possess only specific amounts of energy, based on what observation?
a. | alpha particles were deflected by an electric field |
b. | some α particles were deflected by the gold foil |
c. | most α particles went straight through the gold foil |
d. | the line spectra produced by excited atoms |
e. | a continuous spectra was produced by excited atoms |
d. | the line spectra produced by excited atoms |
14. Bohr's atomic model is referred to as a "quantum" model because
a. | e-s travel in orbits, similar to planets in the solar system |
b. | the mass of e-s is significantly greater than the nucleus |
c. | the nucleus carries an overall negative charge |
d. | each e- can only possess a specific amount of energy |
e. | each proton can only possess a specific amount of energy |
d. | each e- can only possess a specific amount of energy |
15. When an electron goes from a low energy state to a high energy state, what occurs?
a. | light is given off by the electron |
b. | energy is absorbed by the electron |
c. | energy is given off by the electron |
d. | the electron is ejected from the atom |
e. | answers a & c are correct |
b. | energy is absorbed by the electron |
16. Of the following transitions in the Bohr hydrogen atom, which of the following results in the emission of the lowest-energy photon.
a. | n = 1 → n = 6 | d. n = 3 → n = 5 |
b. | n = 5 → n = 1 | e. n = 1 → n = 5 |
c. | n = 6 → n = 1 |
b. | n = 5 → n = 1 |
17. De Broglie first noted the
a. | particle nature of light |
b. | speed of light is 3.00 x 108m/s |
c. | wave nature of matter |
d. | wave nature of light |
e. | Answers a, b & d are correct |
c. | wave nature of matter |
18. An atomic orbital is
a. | the location of an electron in an atom |
b. | the maximum distance that an electron can exist from the nucleus |
c. | where an electron spends 99% of its time |
d. | the region of space in which an e- can be found |
e. | the path that an electron travels in an atom |
d. | the region of space in which an e- can be found |
19. Heisenberg's Uncertainty Principle states that the _________ and ________ of an electron can be measured but not at the same time.
a. | charge, velocity d. charge, spin |
b. | charge, position e. spin, velocity |
c. | position, velocity |
c. | position, velocity |
How many quantum numbers are necessary to designate the ‘location’ of a particular electron in an atom?
a. 3
b. 4
c. 2
d. 1
e. 5
b. 4
n
l
ml
ms
21. Heisenberg’s Uncertainty Principle states that an electron spends ______ of its time inside an atomic orbital.
a. 10%
b. 25%
c. 50%
d. 90%
e. 100%
d. 90%
22. Which quantum number describes the size of the area in which an electron will be found in?
a. n | b. ml | c. ms | d. l | e. d |
a. n
23. How many electrons can exist in an orbital?
a. | A maximum of one electron |
b. | A maximum of two electrons each |
c. | The answer depends on the energy level |
d. | The answer depends on the type of orbital |
e. | The answer depends on the atom |
b. | A maximum of two electrons each |
24. The total number of orbitals in the n = 4 energy level is
a. 128 | b. 64 | c. 32 | d. 16 | e. 8 |
d. 16
The total number of orbitals in an energy level is given by the formula: n2
25. How many orbitals in the n = 3 energy level?
a. 25 | b, 16 | c. 9 | d, 4 | e. 1 |
c. 9
The total number of orbitals in an energy level is given by the formula: n2
26. Which subshell contains only one orbital?
a. 4d | b. 6f | c. 3d | d. 2s | e. 1p |
d. 2s
27. How many orbitals exist in the n = 2 energy level?
a. 1 | b. 4 | c. 8 | d. 10 | e. 12 |
b. 4
28. How many electrons would be found in an atom that has the n = 1 and n = 2 energy levels filled?
a. 2 | b. 4 | c. 6 | d. 8 | e.10 |
e.10
The maximum number of electrons in an orbit of an atom is determined by 2n2
plug n=1 and n=2 into the equation to get 10
29. The n = 1 energy level contains __________ p orbital’s, while all other energy levels contain ________ p orbitals.
3, 3
a. 2, 6 | b. 0, 3 | c. 6, 2 | d. 3, 3 | e. 0, 4 |
b. 0, 3
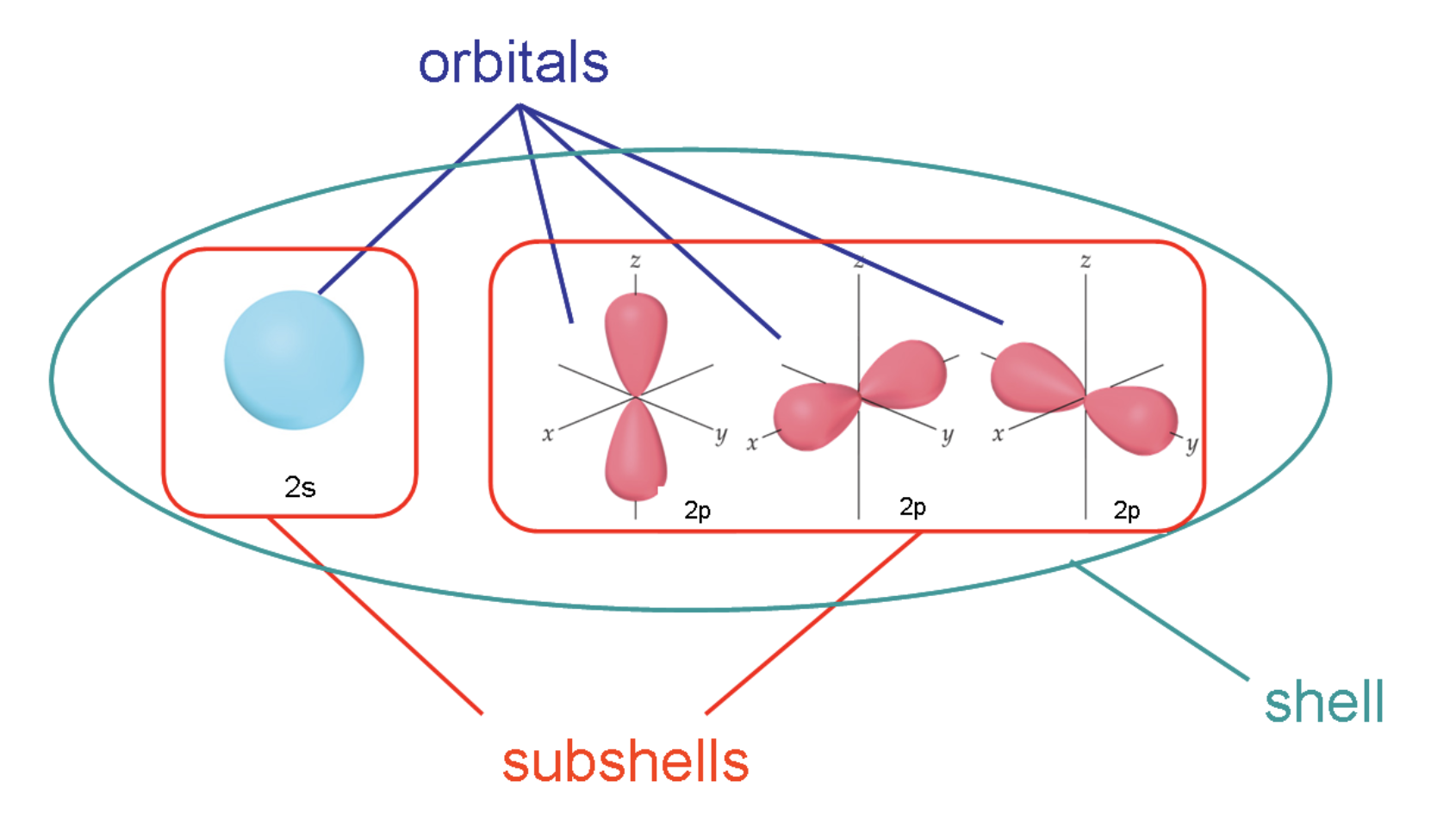
30. The principal quantum number of the first d orbital is ______.
a. 1 | b. 2 | c. 3 | d. 4 | e. 5 |
c. 3
asking ?n
31. The lowest energy level that contains f orbitals is n = ______.
a. 2 | b. 3 | c. 2 | d. 4 | e. 1 |
d. 4
32. The total number of orbitals in any given energy level is _____.
a. n2 | b. 2n | c. 2n2 | d. 2n+1 | e. 2n+2 |
a. n2
33. 3p subshell in the ground state of atomic iodine contains _ e-s.
a. 2 | b. 5 | c. 6 | d. 8 | e. 10 |
c. 6
34. The second shell in the ground state of atomic sulphur contains __ ___electrons.
a. 2 | b. 4 | c. 6 | d. 8 | e. 18 |
d. 8
second shell meaning 2s and 2p…
2 + 6 = 8
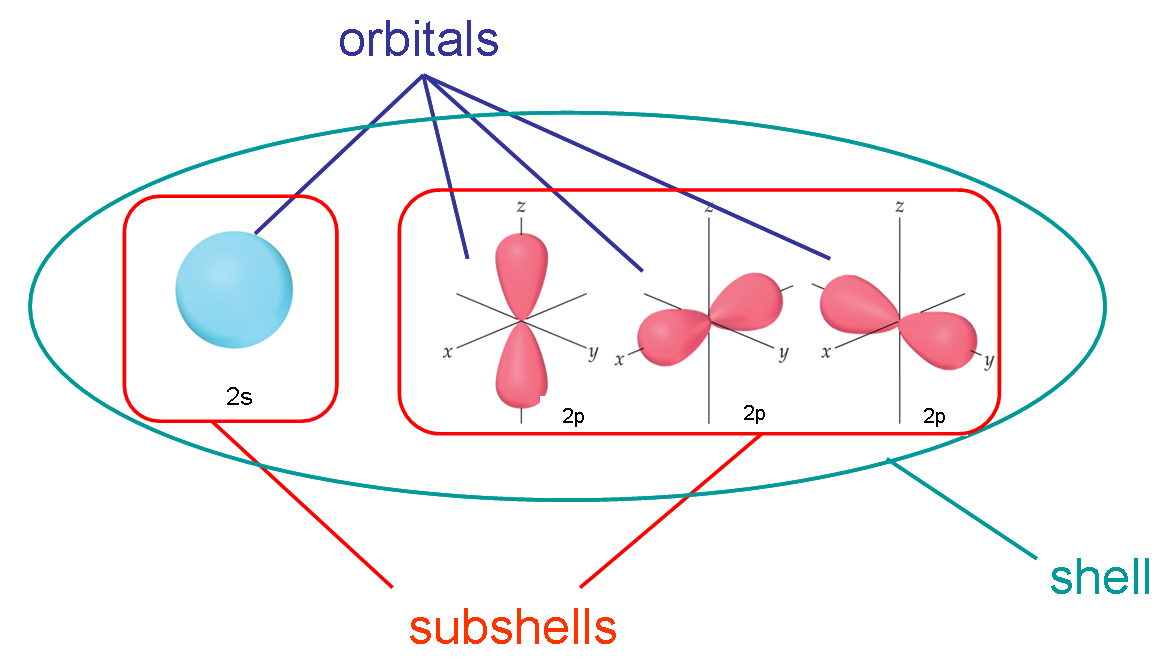
35. At maximum, an f-subshell can hold __________ electrons, a p-subshell can hold __________ electrons, and a d-subshell can hold __________ electrons.
a.14, 10, 6 | c. 14, 8, 2 | e. 18, 10, 6 |
b.14, 6, 10 | d. 18, 6, 10 |
b.14, 6, 10
36. There are __________ unpaired electrons in a ground state sulphur atom.
a. 0 | b. 1 | c. 2 | d. 3 | e. 4 |
c. 2
37. In which period of the periodic table do the elements have a core-electron configuration that is the same as argon.
a. 1st | b. 2nd | c. 3rd | d. 4th | e. 5th |
d. 4th
38. An electron cannot have the quantum numbers n = __________, l = __________, ml = __________.
a. 2,1,-1 | b. 3,1,-1 | c. 1,1,1 | d. 3,2,1 | e, 2,0,0 |
c. 1,1,1
39. Which of the following is not a valid set of four quantum numbers? (n, l, ml, ms )
a.. 4, 0, 0, +1/2 | c. 3, 2, -1, -1/2 | e. 1, 1, 0, +1/2 |
b.2, 1, 0, -1/2 | d. 1, 0, 0, +1/2 |
e. 1, 1, 0, +1/2
40. Which of the following is a valid set of four quantum numbers? (n, l, ml, ms )
a. 4, 0, -1, +1/2 | c. 3, 3, -1, +1/2 | e. 1, 1, 2, +1/2 |
b. 2, 1, 0, -1/2 | d. 1, 0, -1, +1/2 |
b. 2, 1, 0, -1/2
41. The magnetic quantum number of an orbital defines:
a. | The size of the atom. |
b. | The energy level of the outermost electron. |
c. | The shape of the orbital. |
d. | The spatial orientation of the orbital. |
e. | The spin of the electrons in each orbital. |
d. | The spatial orientation of the orbital. |
ml
42. The principal quantum number of an orbital defines:
a. | The size of the atom |
b. | The energy level of the orbital |
c. | The shape of the orbital |
d. | The spatial orientation of the orbital |
e. | The spin of the electrons in each orbital |
b. | The energy level of the orbital |
n
43. Which quantum number defines the shape of an orbital?
a. magnetic | c. azimuthal | e. a & b |
b. principal | d. sigma |
c. azimuthal
l
44. The p atomic orbital has the shape of
a. a sphere | c. a dumb-bell | e. letter ‘t’ |
b. a four leaf clover | d. a pyramid |
c. a dumb-bell
45. A d atomic orbital has a shape resembling
a. a sphere | c. a dumb-bell | e. letter ‘t’ |
b. a four leaf clover | d. a pyramid |
b. a four leaf clover
46. Who hypothesized that only two electrons of opposite spin could occupy an orbital?
a.Schrodinger | c. Aufbau | e. Pauli |
b.Bohr | d. Heisenberg |
e. Pauli
47. Which of the following electron configurations is impossible for an atom, regardless of whether it is in the ground state or an excited state?
a.1s22s22p3 | c. 1s22s12p1 | e. 1s22s22p6 |
b.1s32s12p4 | d. 1s22s12p3 |
b.1s32s12p4
can’t have 3 electrons in an s
48. An electron in a(n) __________ subshell experiences the greatest effective nuclear charge in a many-electron atom.
a. 4f | b. 4p | c. 4d | d. 4s | e. 5s |
d. 4s
Least shielded, highest penetration among the 4th shell orbitals
49. In which orbital does an electron in an atom experience the greatest shielding?
a. 2p | b. 3s | c. 3p | d. 3s | e. 4s |
e. 4s
The greatest shielding occurs in orbitals that are further away from the nucleus and have a higher principal quantum number.
50. As you move from left to right across the periodic table, an element’s atomic radius __________.
a.increases | d. is randomly assigned |
b.decreases | e. depends on the period |
c.remains unchanged |
b.decreases
51. Arrange the following elements from highest to lowest ionization energy:
a, Be, Mg, Ca, Rb, Y | d. Rb, Sr, Ca, Mg, Be |
b. Be, Mg, Ca, Rb, Sr | e. Be, Mg, Ca, Sr, Rb |
c. Rb, Sr, Ca, Be, Mg |
e. . Be, Mg, Ca, Sr, Rb
the amount of energy required to remove an electron from an isolated atom or molecule.
Ionization energy decreases as you move down a group:
As you move down a group, the atomic radius increases, and the outer electrons are farther from the nucleus, resulting in less attraction and easier removal.Ionization energy increases as you move across a period:
As you move from left to right across a period, the nuclear charge increases, but the electrons are added to the same energy level, leading to a stronger attraction and harder removal.
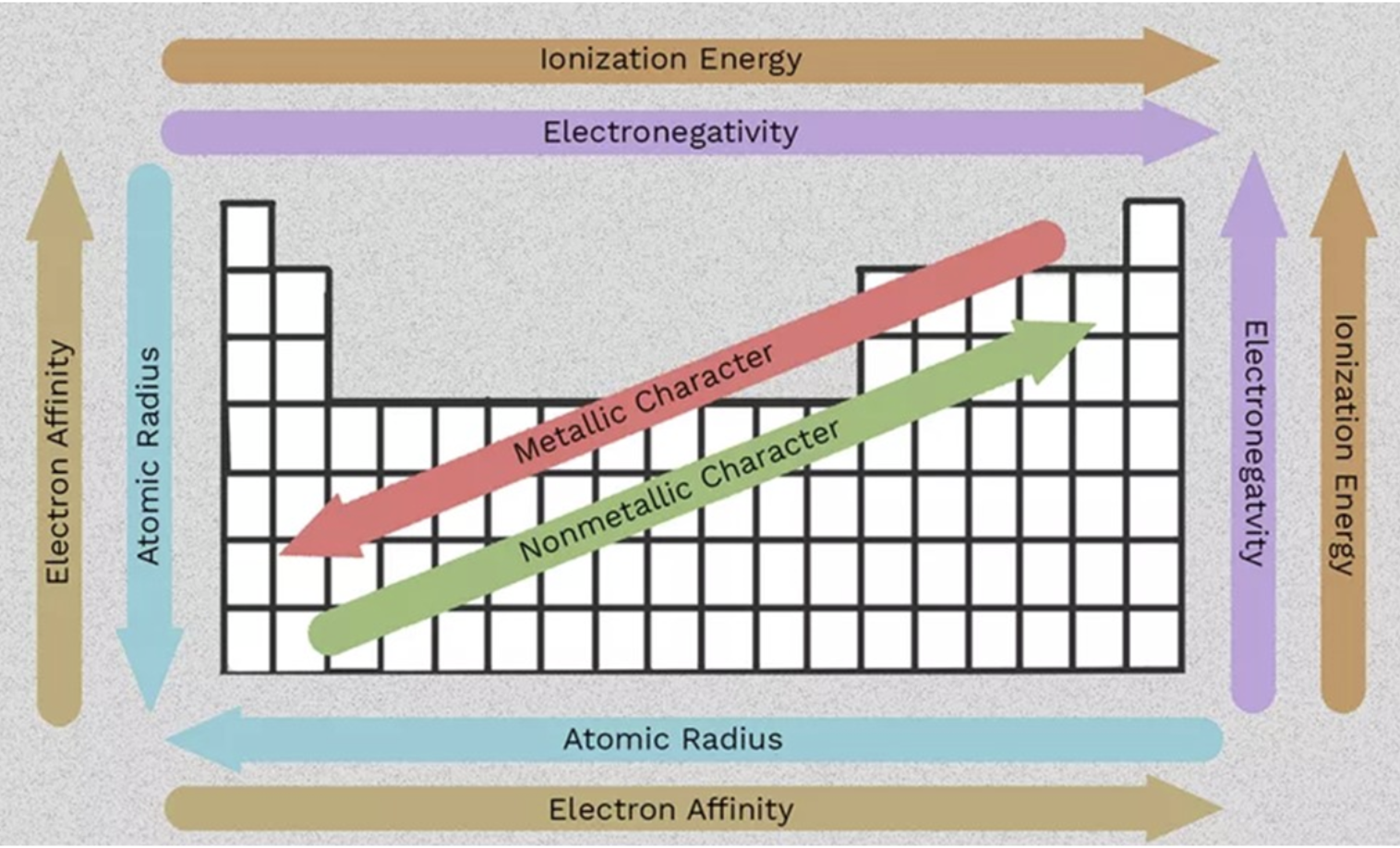
52. Arrange the following elements in order of increasing effective nuclear charge:
a. Li, B, C, Be, O | d. C, N, O, Li, Be |
b.Al, Si, P, Na, Mg | e. S, P, Si, Al, Mg |
c.Be, B, C, N, F |
c.Be, B, C, N, F
Electrons in higher energy levels (such as 4s) are shielded more by the inner electrons because they are farther from the nucleus.
Zeff increases L→R across a period, but the core structure is the same for all (the noble gas of the n-1 period)
53. Of Ru, Cs, As, Pb and Br, which would you expect to have the greatest electron affinity?
a. Cs | b. Ru | c. . Pb | d. Br | e. As |
d. Br
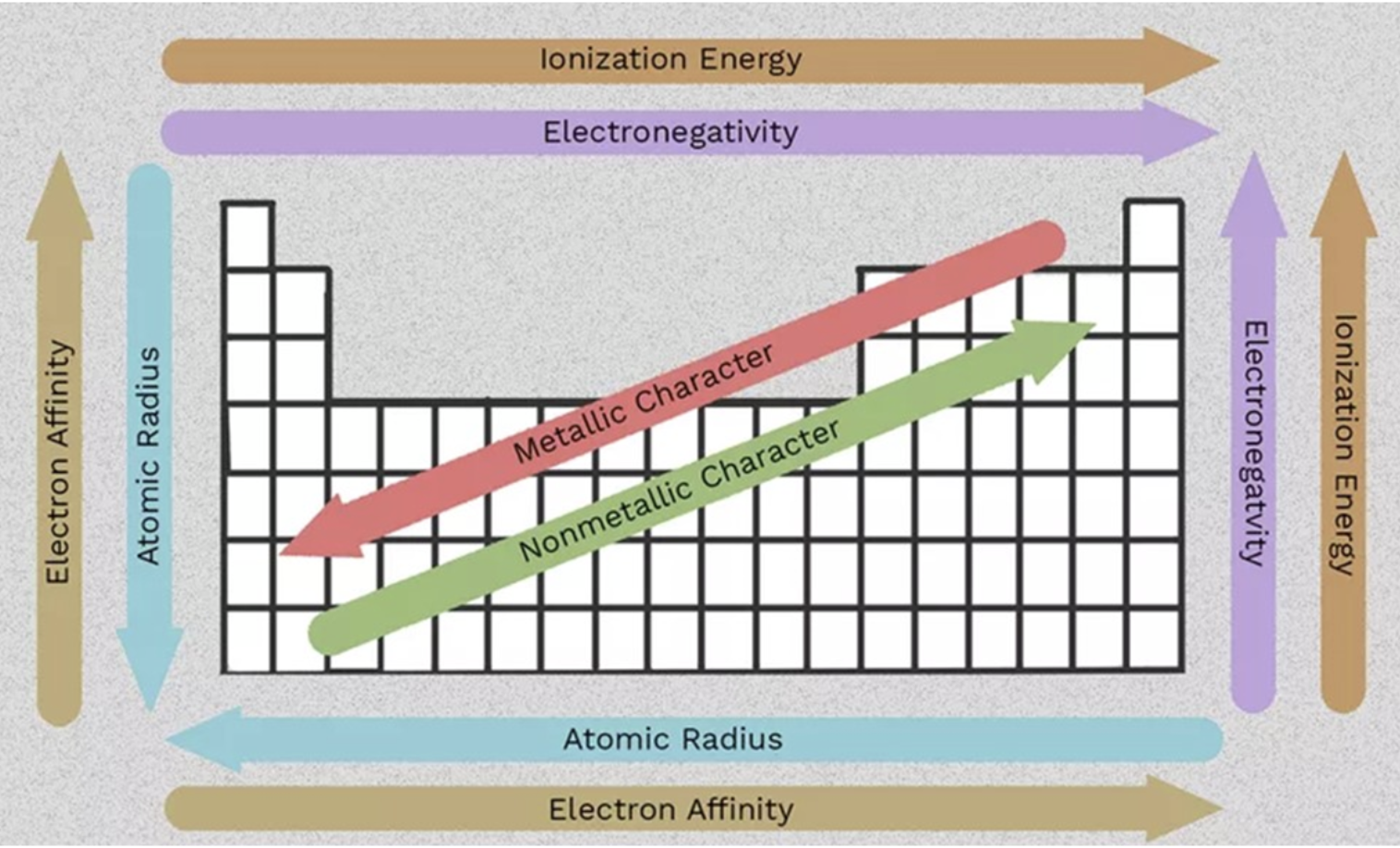
*Of the following types of electromagnetic radiation, which has the shortest wavelength
a. infrared light
b. ultraviolet light
c. green light
d. red light
e. orange light
b. ultraviolet light
*The lines in the line spectrum of an atom results from
a. energy absorbed by electrons dropping towards their ground state.
b. energy absorbed by electrons jumping to a higher energy level.
c. energy released by electrons jumping to a higher energy level.
d. energy released by electrons dropping towards their grounds state.
e, none of the above.
d. energy released by electrons dropping towards their grounds state.
d. each electron can only possess a specific amount of energy.
c. position, momentum
*The fact that electrons can only exist at specific allowable energy levels in an atom can be explained if we apply the wave/particle duality to electrons. Which of the following best describes an electron in the quantum mechanical model?
a. Particles that randomly circle the nucleus.
b. A particle that circles the nucleus in orbits like planets around the sun.
c. A standing wave like the vibration of a guitar string.
d. None of the above
c. A standing wave like the vibration of a guitar string.
*Which quantum number describes the orientation in space of a given orbital (subshell)
a. n
b. ml
c. ms
d. l
e. d
b. ml
n= mainshell
l = shape / suborbital
ml = orientation in space
ms = spin
*Ignoring the rules for building or filling atoms with electrons, how many electrons would be found in an atom that has the first three mainshells (n=1, n=2, and n=3) filled?
a. 2
b. 10
c. 18
d. 28
e. 36
d. 28
Max electrons in a shell=2n2
n=1 (the first shell) can hold up to 2 electrons.
n=2 (the second shell) can hold up to 8 electrons.
n=3 (the third shell) can hold up to 18 electrons.
So, the total number of electrons in an atom with these three shells filled is:
2 (n=1) + 8 (n=2) + 18 (n=3) = 28 electrons.
*The principal quantum number of the first d orbital is ____ (another way to ask is: in which mainshell are the d orbitals first posssible
a. 1
b. 2
c. 3
d. 4
e. 5
c. 3
The d orbitals start at n = 3.
d. 4
*There are ____ unpaied electrons in a ground state selenium (34Se) atom
a. 0
b. 1
c. 2
d. 3
e. 4
c. 2
Which of the following is not a valid set of four quantum numbers (n,l,ml,ms)?
a. 4,0,0,+1/2
b. 2,1,0,-1/2
c. 3,2,-1.-1/2
d. 1,0,0,+1/2
e. 1,1,0,+1/2
e. 1,1,0,+1/2
l cannot equal 1 since l={n-1}… 0
*Which sublevel designations describe a spherical shaped orbital
a. 4p
b. 3d
c. 2s
d. 5f
c. 2s

*The diagrams below represent which orbitals
a. an s and a p
b. a p and an f
c. a p and a d
d. a d and an f
d. a d and an f
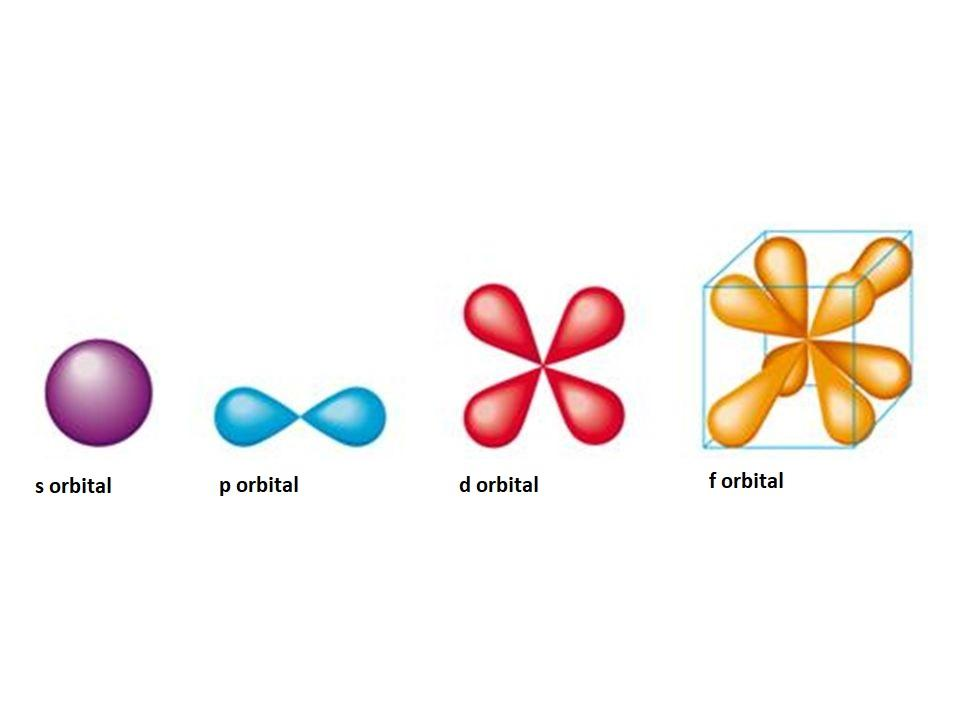
*A 4p atomic orbital has the shape of a
a. a sphere
b. a torus
c. a dumbbell
d. double dumbbells (perpendicular)
e. an egg
c. a dumbbell
*An electron in a(n) _______ subshell experiences the greatest effective nuclear charge in a many electron atom (i.e. in which subshell can an electron penetrate closest to the nucleus?)
a. 4f
b. 5d
c. 6d
d. 7s
e. 5p
e. 5p
The order of penetration follows this trend:
s>p>d>f
5p (Choice e) → P-orbitals penetrate better than d and f, and since 5p is closer to the nucleus than 7s, it experiences a higher Z_eff.
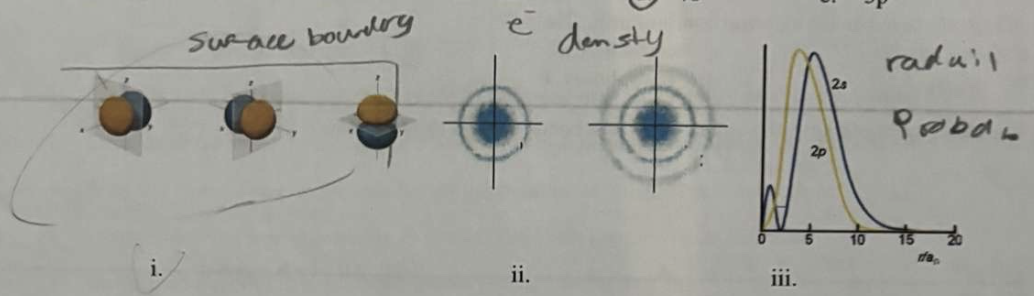
(left to right i,ii,iii)
*Of the representations above, what is the name for (iii)?
a. surface boundary layer
b. bell curve
c. radial probability
d. electron dot
e. electron density
c. radial probability
pic is how it actually looked on the test…
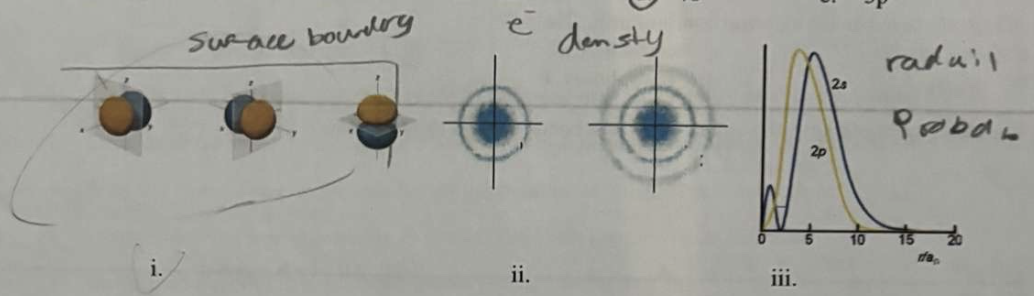
(left to right (ii),(i),(iii)) (read carefully lol)
*Of the representations above, what is the name for (i)?
a. surface boundary layer
b. bell curve
c. radial probability
d. electron dot
e. electron density
a. surface boundary layer
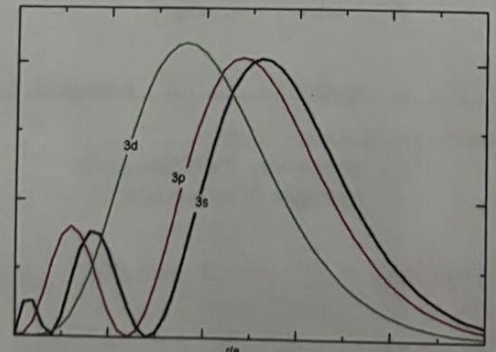
*In the following graph, it would appear that the large hump in the 3d graphs shows that a 3d electron would spend more time closer to the nucleus, and so should have less energy and be filled before either the 3p or 3s
a. the p shell has a greater percent probability area under the line
b. the d shell is the furthest away from the nucleus
c. penetration to the nucleus
d. the s shell contains fewer electrons
e. ionization energy is greater in the d shell
c. penetration to the nucleus

b.1s22s22p63s1

a. 1s22s22p6
*Which element has the electron configuration [Ne]3s2?
a. sodium, Na
b. argon, Ar
c. calcium, Ca
d. phosphorus, p
e. magnesium, Mg
e. magnesium, Mg
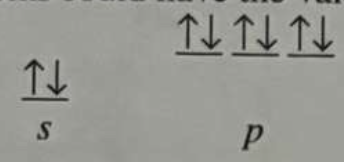
*Which atoms could have the valence electron configuration shown in the image:
a. N3-
b. O2-
c. Ar
d. Cl1-
e. All of the above
e. All of the above
Which group in a period will have the highest ionization energies and lowest (most negative) electron affinities?
a. alkaline earth metals
b. metalloids
c. halogens
d. transition metals
e. noble gases
e. noble gases
*Which properties is accounted for by the fact that unpaired electrons in an element have the same spin
a. ability to reflect light
b. ability to be drawn into a wire
c. paramagnetism
d. reactivity with oxygen
c. paramagnetism
*Which of these is an s-block element
a. oxygen, O
b. argon, Ar
c. cesium, Cs
d. potassium, K
d. potassium, K
*Which pairs of elements have valance electron configurations of s2p3?
a. fluorine, F and chlorine, Cl
b. boron, B, and sodium, Na
c. phosphorus, P, and nitrogen, N
d. hydrogen, H, cesium, Cs
c. phosphorus, P, and nitrogen, N
*The calcium ion is isoelectronic with
a. neon, Ne
b. sodium, Na
c. rubidium, Rb
d. argon, Ar
e. krypton, Kr
d. argon, Ar
isoelectronic - two atoms, ions, or molecules that have the same electronic structure and the same number of valence electrons.
Which of the following manganese describe 25Mn
i. paramagnetic
ii. diamagnetic
iii, transition metal
iv. representative metal
v. p-block
vi. d-block
a. i., iii., and v
b. ii, iii, and v
c. i, iii, and vi
d. ii, iii, vi
e. ii, iv, and v
c. i, iii, and vi
i. paramagnetic
iii, transition metal
vi. d-block
**Matching
At the end of the 1800’s, the model of light as a wave into question. Match the names of the scientists with the appropriate statement. Each name may be used MORE THAN ONCE or NOT AT ALL
a. Maxwell
b. Planck
c. Einstein
d. Pauli
e. Bohr
f. de Broglie
g. Heisenberg
h. Schrodinger
I. Hund
j. Mendeleev
‘Two things can’t be in the same place at the same time’ is one way of communicating his idea of electrons
developed the wave function: the calculus behind quantum mechanics, the modern view of the atom
Won the Nobel Prize in physics for his explanation of the photoelectric effect
Determined that is impossible to predict the exact location of an electron
Developed a model to explain the atomic spectra of hydrogen
Proposed that all matter exhibits both wave and particle properties; which one predominates depends on the size of the particle
Gave us the idea of photons: light as packets of energy
Solved the ‘ultraviolet catastrophe’ using E=hv
D Pauli
H Schrodinger
C Einstein
G Heisenberg
E Bohr
F de Broglie
C Einstein
B Planck
Match the following scientists and scientific findings.
a. | Compared the structure of an atom to a plum pudding |
b. | Compared the structure of an atom to planets in motion around a central body |
c. | Compared the particles that make up matter to small hard spheres |
d. | Compared the orbits in which electrons exist to planetary orbits, also called energy levels |
e. | Light travels as packets of energy. |
1. Dalton
2. Thomson
3. Bohr
4. Rutherford
5. Einstein
C
A
D
B
E
Match the following terms with their definitions.
a. | Light that is absorbed by electrons of a specific element |
b. | The shortest distance between equivalent points on a continuous wave |
c. | Light that is released by electrons of a specific element |
d. | A measure of the intensity of a wave |
e. | The number of wave cycles that pass a given point in a unit of time |
6. Emission spectrum
7. Wavelength
8. Frequency
9. Absorption Spectrum
10. Amplitude
C
B
E
A
D
Match the following experiments with their discovery.
a. | Led to the discovery of the nucleus | c. | Led to the discovery that matter acts like a wave |
b. | Led to the discovery that light acts as a particle | d. | Led to the discovery of the electron |
11. Cathode ray tube
12. Electron diffraction
13. Gold foil expmT
14. Photoelectric effect
D
C
A
B
Match the following terms with the correct definition.
a. | describes an orbital’s shape |
b. | specifies the relative size of an orbital |
c. | describes an orbital’s orientation in space |
d. | specifies the orientation of the axis on which the electron is spinning |
15. l
16. ml
17. n
18. ms
A
C
B
D
Match the following terms with the correct definition.
a. | Related a wave function to a region of space called an atomic orbital |
b. | Mathematically showed that the position and momentum of a particle cannot be determined with certainty |
c. | Showed that wave functions could be used to determine the probability of finding an e-within a region of space |
d. | Stated that a maximum of two electrons can occupy an orbital |
e. | Proposed the theory of matter waves |
Pauli
DeBroglie
Born*
Schrödinger
Heisenberg
D
E
C
A
B
Match the following terms with the correct definition.
a. | the principle that no two identical particles can occupy the same quantum state at the same time |
b. | region of high probability in which an electron will be found |
c. | electrons fill orbitals starting at the lowest available |
d. | a maximum number of electrons will try occupy orbitals singly |
e. | the arrangement of electrons in an atom |
24. Atomic orbital
25. Hund’s Rule
26. Electron configuration
27. Aufbau principle
28. Pauli exclusion principle
B
D
E
C
A
Compare and contrast:
a) Thomson’s model and Rutherford’s
J.J. Thomson’s experiments with cathode ray tubes provided groundbreaking evidence for the existence of subatomic particles. In these sealed glass tubes with most of the air evacuated, a high voltage applied across two electrodes caused a glowing beam to flow from the negatively charged cathode to the positively charged anode.
To determine the nature of the cathode ray, Thomson placed oppositely charged electric plates around it. The ray was deflected away from the negative plate and toward the positive one, indicating that it was composed of negatively charged particles. He also introduced a magnetic field, which similarly deflected the ray, confirming that the ray was made up of particles and not light, and it had mass and charge. With this information, Thompson was able to determine the mass-to-charge ratio and discovered the particles were much smaller than any known atom, (they were 1/1800 the mass of Hydrogen, the smallest known atom)proving they were subatomic particles. He concluded that these particles, which could conduct electricity and where thus called electrons, were a fundamental part of all elements
Since atoms are neutral, Thomson reasoned that there must be a positive charge to balance the electrons. Though he couldn’t detect it directly, he proposed the Plum Pudding Model, where electrons were embedded in a diffuse, positively charged field.
Rutherford discovered 3 types of radiation emissions (alpha, beta, gamma) which are energetic particles that can penetrate matter. Alpha particles are positively charged and large compared to beta particles and electrons. Beta particles are very energetic and negatively charged (later proven to be electrons) with a very small mass. Gamma rays are neutrally charged, have no mass and are just pure energy. Rutherford conducted a gold foil experiment to test Thomson’s theory, and emitted positive, high-speed alpha particles toward a thin piece of gold, expecting them to go straight through as the electrons should have no effect given how spread out they are in Thompson’s model.
98% went straight, 2% went straight and then deflected at large angles, and 0.005% bounced off backwards. He concluded that the atom was mostly space since almost all went through, but there was a dense particle (we know as the nucleus) that is small in volume but large in mass. Rutherford came up with the beehive model that has very small -ve charged electrons zipping around a dense nucleus containing protons and neutrons.
Compare and contrast:
b) Bohr’s model to the quantum mechanical model
Bohr’s model and the quantum mechanical model both describe atomic structure, but the latter provides a much more detailed and accurate representation. Bohr’s model, proposed in 1913, assumes that electrons move in fixed circular orbits around the nucleus at specific energy levels. These orbits are quantized, meaning electrons can only exist at certain distances from the nucleus without radiating energy. When electrons jump between these levels, they absorb or emit photons of specific wavelengths, explaining atomic emission spectra. However, Bohr’s model only works well for one-electron systems and fails to account for the complexities of multi-electron atoms, electron-electron interactions, and the fine structure of spectral lines.
The quantum mechanical model, based on Schrödinger’s wave equation, replaces Bohr’s fixed orbits with probabilistic electron distributions called orbitals—three-dimensional regions where electrons are likely to be found. Heisenberg’s uncertainty principle states that an electron’s exact position and momentum cannot be simultaneously known, so quantum mechanics instead describes electron probability densities using wave functions (Ψ). Atomic orbitals enclose 90% of the electron probability and come in distinct shapes: s (spherical), p (dumbbell), d (double-dumbbell), and f (double-double dumbbell).
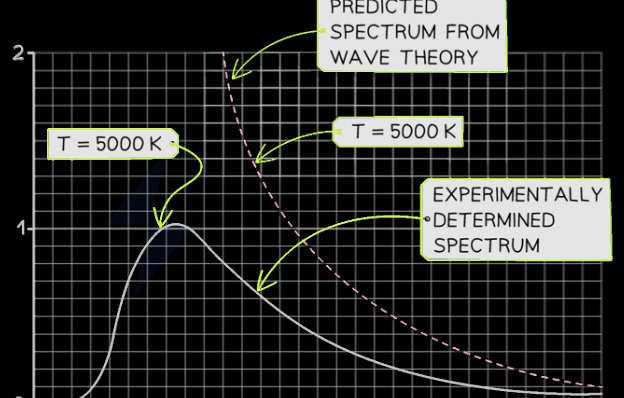
2. Briefly describe the three observations that brought into question the notion of light as a wave and how they changed the model of light.
The three observations that brought into question the notion of light as a wave are blackbody radiation, the photoelectric effect, and atomic line spectra.
A blackbody is an idealized object that absorbs all electromagnetic radiation that falls on it. When heated, it emits radiation, starting with low-energy infrared (IR) and progressing through the visible spectrum to ultraviolet (UV) as the temperature rises (electrons gain energy and release it as light— glowing). According to thermal equilibrium, a blackbody should re-emit all absorbed wavelengths as it heats up. Physicists used the wave nature of light to predict that as wavelength decreases, the emitted energy should increase indefinitely, leading to an unrealistic surge in ultraviolet (UV) radiation. However, experimental data showed that energy emission actually peaks and then drops off at shorter wavelengths. This led to the ultraviolet catastrophe, as the wave nature of light did not support the experimental evidence or explain how an object glows when its temperature increases.
The photoelectric effect occurs when light shines on a metal’s surface, causing it to emit electrons. Classical wave theory suggested that light’s energy comes from its amplitude, meaning any color of light should cause electron emission if the metal absorbs enough energy, with brighter light ejecting electrons with greater energy and dim light causing a delay. However, it was observed that only light above a certain frequency, known as the threshold frequency, could cause electron emission, regardless of intensity. Light with a frequency above this threshold would immediately emit electrons, even if dim, but with fewer electrons than brighter light of the same frequency. This showed that frequency, not brightness, determined the energy of the emitted electrons, revealing that wave theory couldn’t explain the photoelectric effect.
When atoms or molecules absorb energy, they often release it as light energy. A spectroscope, which functions like a prism, breaks light into its visible spectrum. Classical physics predicted that the emitted light from elements would form a continuous spectrum (ROYGBIV), but experiments showed that pure elements produce distinct lines of specific colors, not a continuous range. This observation, known as atomic line spectra, contradicted the wave theory of light, as it couldn’t explain why elements emitted only certain wavelengths. This led to the conclusion that the energy levels of atoms are quantized, with electrons transitioning between specific energy levels, prompting a shift to a new model of light.
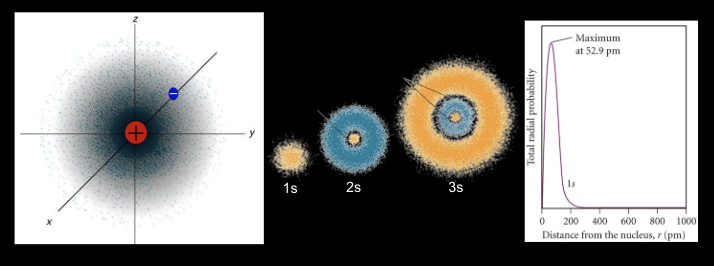
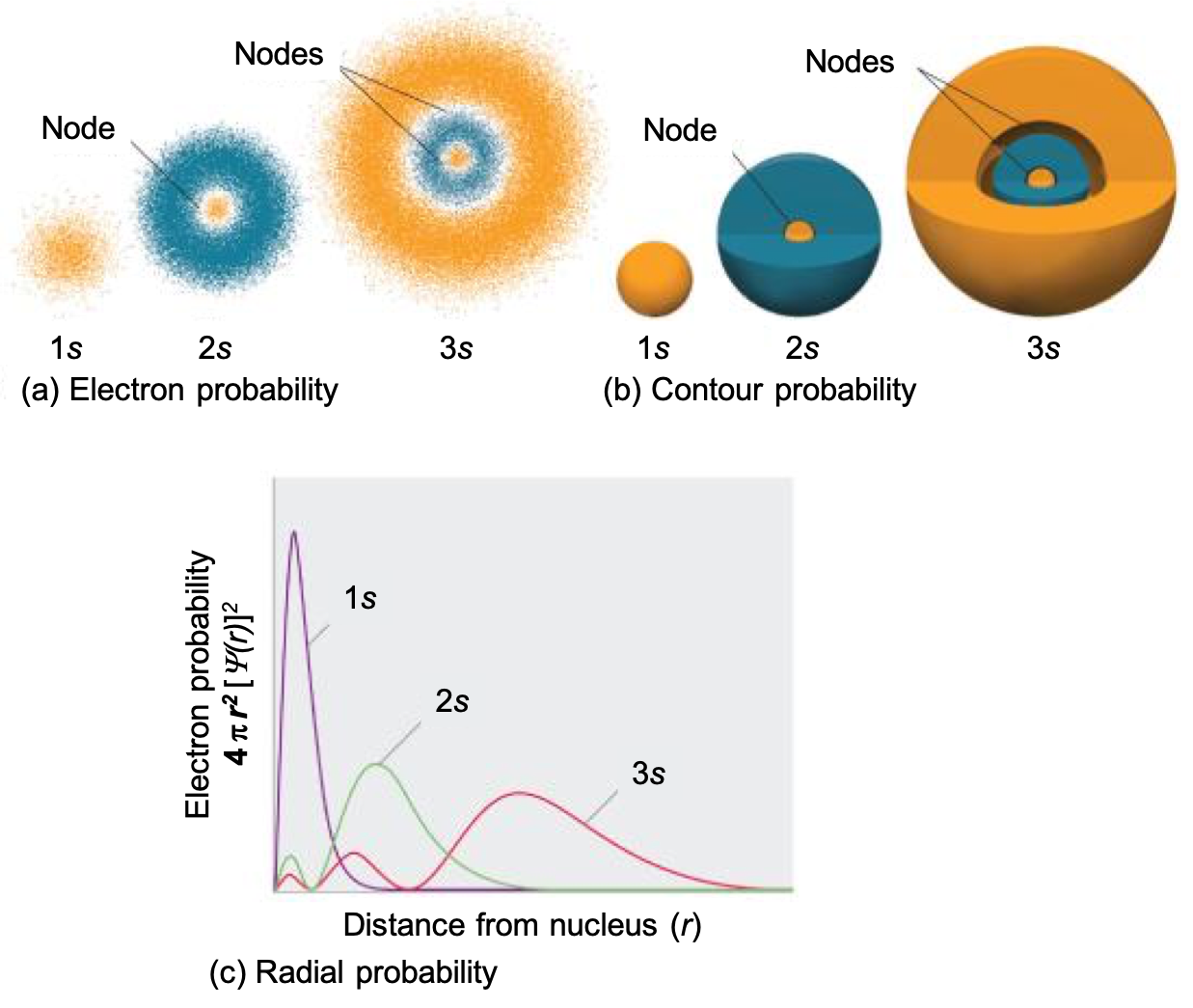
3. Differentiate between electron density diagrams, boundary surface layer diagrams and radial probability diagrams.
Electron Density Diagram: Shows the probability of finding an electron in space around the nucleus. Darker areas indicate higher probability, providing a continuous view of electron distribution but are hard to draw.
Boundary Surface Diagram: Defines a region where the electron is most likely found (typically 90% probability). Used to visualize the shape, size, and orientation of orbitals (s, p, d, f).
Radial Probability Diagram: A graph showing the likelihood of finding an electron at a specific distance from the nucleus. Peaks indicate the most probable electron position, considering both distance and spherical volume.
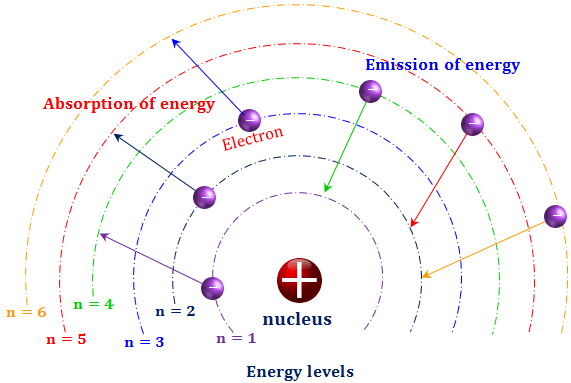
4. Explain how electrons absorb or release energy when they move between energy levels. Use a diagram as part of your explanation.
When electrons absorb energy, they move to a higher energy level (excited state). When electrons release energy, they fall back closer to the ground state. The particles they absorb/emit are called photons — the smallest possible packets of electromagnetic energy. If an electron absorbs a photon to go up an energy level, it will emit the same photon to go back down. The difference in energy between the states corresponds to the frequency of radiation in the atomic line spectra.
5. Apply the Pauli Exclusion Principle to describe the quantum numbers of both electrons in a helium atom.
For the helium atom, the first three quantum numbers for the two electrons are the same. n=1, l = 0, and ml = 0. But they are differentiated by their spin (ms) — one electron has a spin of a +1/2 while the other has a spin of a −1/2 , making them unique and distinct.
6. Write the correct electron figuration and condensed electron configuration for a silver atom (47Ag), and draw the energy level diagram.
1s22s22p63s23p64s23d104p65s14d10 — remember the exception,
[Kr]4d105s1
![<p><span style="font-size: inherit">1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>2</sup>3p<sup>6</sup>4s<sup>2</sup>3d<sup>10</sup>4p<sup>6</sup>5s<sup>1</sup>4d<sup>10</sup> — remember the exception,</span></p><p>[Kr]4d<sup>10</sup>5s<sup>1</sup></p>](https://knowt-user-attachments.s3.amazonaws.com/cdc5cf75-efd0-4d28-b98d-b8b1af77b0fd.jpg)
7. Provide a set of quantum numbers for each of the last two electrons in a rubidium atom (if you were building the atom).
37Rb = [Kr] 5s¹ or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1.
for 5s1:
n= 5
l = {n-1} = 4,3,2,1,0 (…f,p,d,s) → 0
ml= ±{l} = 0
ms= +1/2
(5,0,0,+1/2)
for 4p6
n=4
l = {n-1} = 3,2,1,0 (f,p,d,s) → 2
ml= ±{l} = +2,+1,0,-1,-2 = -1
ms= -1/2
(4,2,-1,-1/2)
8. Explain what is incorrect with respect to the following set of quantum numbers:
a) n = 3, l = 3, ml = -1
b) n = 2, l = 1, ml = +2
a) n=3, so l ≠ 3 since l = {n-1}, ∴l should = {2,1,0},
b) l=1, so ml ≠ +2 since l can only exist as ±{l}, ∴ {+1,0,-1}
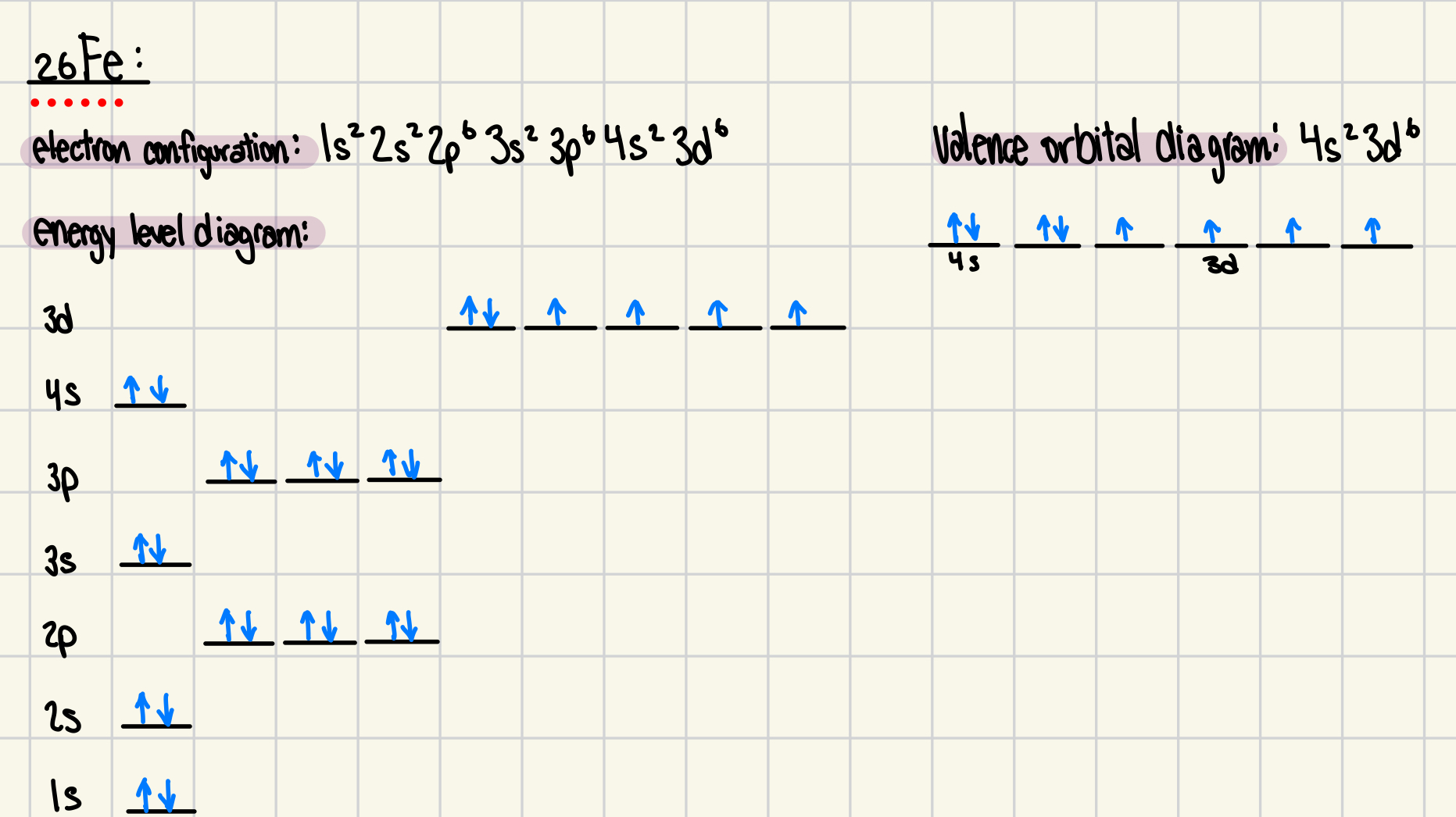
9. Provide the energy diagram, valence orbital diagram and electron configuration for the element iron, 26Fe. Referring to the diagrams, justify the need for the Aufbau principle and Hund’s rule.
Electron configuration: 1s22s22p63s23p64s23d6
Energy Level Diagram:
Valance orbital diagram:
Aufbau Principle
The Aufbau principle states that electrons fill orbitals in order of increasing energy.
Without this rule, electrons could be placed in higher-energy orbitals before lower-energy ones, leading to an unstable configuration.
Hund’s Rule
Hund’s rule states that electrons occupy empty orbitals of the same energy (degenerate orbitals) before pairing.
In Fe’s 3d orbitals, five electrons are first placed in separate orbitals before the sixth pairs.
This minimizes electron-electron repulsion, increasing stability.
Without Hund’s rule, electrons would pair up prematurely, leading to higher repulsion and less stability.
10.
a) What makes one atom diamagnetic and another paramagnetic?
b) Write the expected condensed electron configuration for 46Pd and explain how experimental evidence that shows palladium to be diamagnetic would change this configuration..
Diamagnetic atoms have all their electrons paired in their orbitals. Since paired electrons have opposite spins, their magnetic fields cancel out, making the atom not attracted to a magnetic field.
Paramagnetic atoms have at least one unpaired electron, which means their magnetic fields do not cancel out. This makes the atom attracted to a magnetic field. ( an odd number of e- will always be paramagnetic)
note: not all atoms with with even numbers of e- will be diamagnetic
b) The expected condensed electron configuration for palladium follows the Aufbau principle: [Kr]5s24d8
Diamagnetic substances have all their electrons paired, meaning there are no unpaired electrons in their orbitals. The expected configuration suggests that the 4d subshell is not fully filled, likely leading to unpaired electrons. However, experimental evidence shows that palladium is diamagnetic, meaning all electrons must be paired.
To account for this, the actual electron configuration of palladium is: [Kr]4d10
Here, instead of having two electrons in the 5s orbital and eight in the 4d orbital, both 5s electrons are promoted to the 4d orbital, completely filling it.
11. What would the electron configuration for Fe3+ be?
The electron configuration for the Fe3+ ion is 1s2 2s2 2p6 3s2 3p6 3d5
Fe itself is 1s22s22p63s23p64s23d6
Iron loses three electrons to form Fe+.
The electrons are removed in this order: first from the 4s orbital, then from the 3d orbital.