2. ELECTROLYSIS
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
52 Terms
What is electrolysis?
breaking down an ionic compound into its elements using electricity
Where does electrolysis occur?
in an electrolytic cell
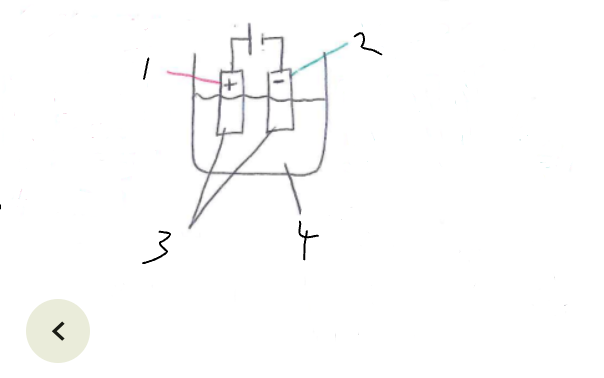
What part of the electrolytic cell is 1
anode
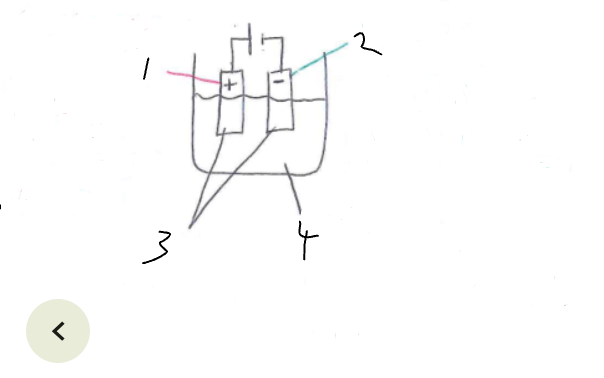
What part of the electrolytic cell is 2
cathode
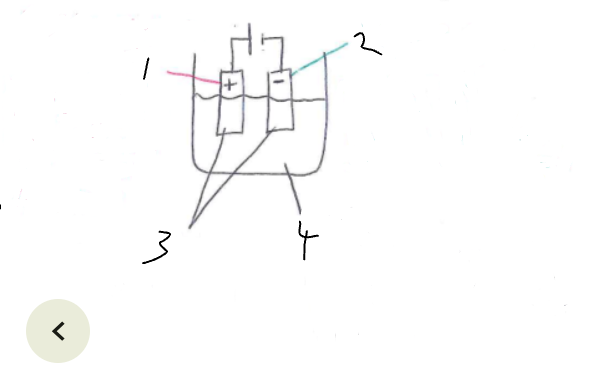
What part of the electrolytic cell is 3
electrodes
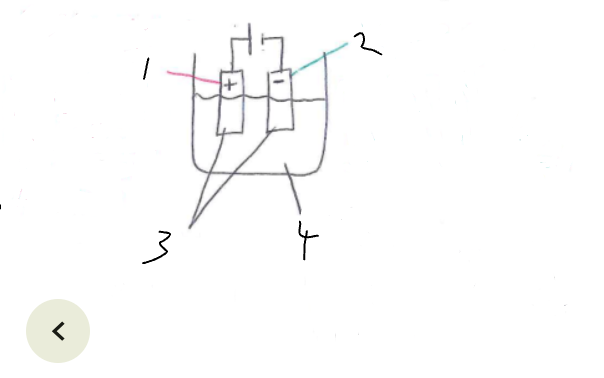
What part of the electrolytic cell is 4
electrolyte
What is an anode?
positive electrode
What happens at the anode?
negative ions are attracted to the anode and lose electrons
What is a cathode?
negative electrode
What happens at the cathode?
positive ions are attacted to the cathode and gain electrons
What is special about electrodes?
they are inert (unreactive)
What is electrolyte?
molten ionic compounds or solutions that conduct electricity, so ions are able to move
What is oxidation?
loss of electrons
Where does oxidation occur?
at the anode
What is reduction?
gain of electrons
Where does reduction occur?
at the cathode
What is an easy way to remember oxidation and reduction?
OIL RIG = Oxidation is Loss, Reduction is Gain
What is an easy way to remember which ion goes to which electrode?
PANIC = Positive Anode, Negative Is Cathode
What happens in the electrolysis of molten compounds?
the metal will be produced at the cathode and the non-metal at the anode
What happens at the anode at the electrolysis of molten lead bromide?
negative bromide ions are attracted to the anode and lose electrons forming bromine atoms
two bromine atoms join to form bromine gas
What happens to the bromine ions in the electrolysis of molten lead bromide?
bromine ions are reduced
What is the equation for the electrolysis of molten lead bromide at the anode?
2Br-(l) → Br2 (g) + 2e-
2Br-(l) - 2e- → Br2(g)
What happens at the cathode at the electrolysis of molten lead bromide?
Positive lead ions are attracted to the cathode and gain electrons forming lead
What happens to the lead ions in the electrolysis of molten lead bromide?
lead ions are oxidised
What is the equation for the electrolysis of molten lead bromide at the cathode?
Pb2+(l) + 2c- → Pb(s)
When do metals have to be extracted from molten compounds?
when the metal is more reactive than carbon so can’t be reduced by it
What is a disadvantage of extracting metals from molten compounds?
expensive because a lot of energy is needed to melt compiunds and produce electricity
What is aluminium ore?
bauxite
What is the formula for bauxite?
Al2O3
What is aluminium oxide dissolved in?
cryolite because it lowers the boiling point of bauxite
What happens at the anode in the extraction of aluminium?
O2- ions are attracted to the anode and lose two electrons forming oxyggen atoms
two oxygen atoms join to make oxygen case
What is the equation for the extraction of aluminium at the anode?
2O-2(l) → O2(g) + 4e-
2O-2(l) - 4e- → O2(g)
What causes the anodes to wear away during the extraction of aluminium at the anode?
oxygen produced her reacts with the graphite producing CO2
What happens at the cathode in the extraction of aluminium?
Al3+ ions attracted to the cathode gain 3 electrons
What is the equation for the extraction of aluminium at the cathode?
Al3+ (l) + 3e- → Al (l)
What ions are contained in solution?
H+, OH-
What does the ions discharged at each electrode depend on?
the reactivity of the elements involved
What happens in the Electrolysis of Aqueous Solutions at the cathode?
H2 produced unless the metal is less reactive than hydrogen
What happens in the electrolysis of Aqueous Solutions at the anode?
O2 produced unless solution contains halide ions
What happens in the electrolysis of lead sulphate solution at the cathode?
Pb2+ and H+ both attracted to cathode
H+ less active so hydrogen gas forms
What is the equation for the electrolysis of lead sulphate solution at the cathode?
2H+(aq) + 2e- →H2(g)
What happens in the electrolysis of lead sulphate solution at the anode?
OH- and SO4-2 both attracted to anode oxygen gas is formed (no halide ions present)
What is the equation for the electrolysis of lead sulphate solution at the anode?
4OH-(aq) → O2(g) + 2H2O(l) + 4e-
4OH-(aq) - 4e- → O2(g) + 2H2O(l)
What is brine?
concentrated sodium chloride solution
What happens in the electrolysis of brine at the cathode?
H+ and Na+ are both attracted to the cathode
H+ gains an electrons forming hydrogen atoms
2 hydrogen atoms bond to form hydrogen gas
What is the equation for the electrolysis of brine at the cathode?
2H+(aq) + 2e- → H2(g)
What happens in the electrolysis of brine at the anode?
OH- and Cl- are both attracted to the anode
Cl- loses an electron to form chlorine atoms (oxidation
Two chlorine atoms bond to form chlorine gas
What is the equation for the electrolysis of brine at the anode?
2Cl-(aq) - 2e- → Cl2(g)
2Cl-(aq) → Cl2(g) + 2e-
What happens to the remaining Na+ and OH- in brine after electrolysis?
they remain dissolved in solution and bond forming sodium hydroxide
What is H2 used for?
make HCl
alternative fuel
What is Cl2 used for?
make bleach
kill bacteria
What is NaOH used for?
make bleach
make paper