U3 Chem H. - Fung
1/27
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
28 Terms
Photon =
a particle that holds a quantum of light/electromagnetic radiation
Quantum =
Quanta =
Single unit of energy (quanta is the plural form)
Amplitude =
Wavelength =
Frequency =
(include units + sketch examples)
height of wave from origin to peak (m)
distance from crest to crest (m)
how fast the wave oscillates (Hz)
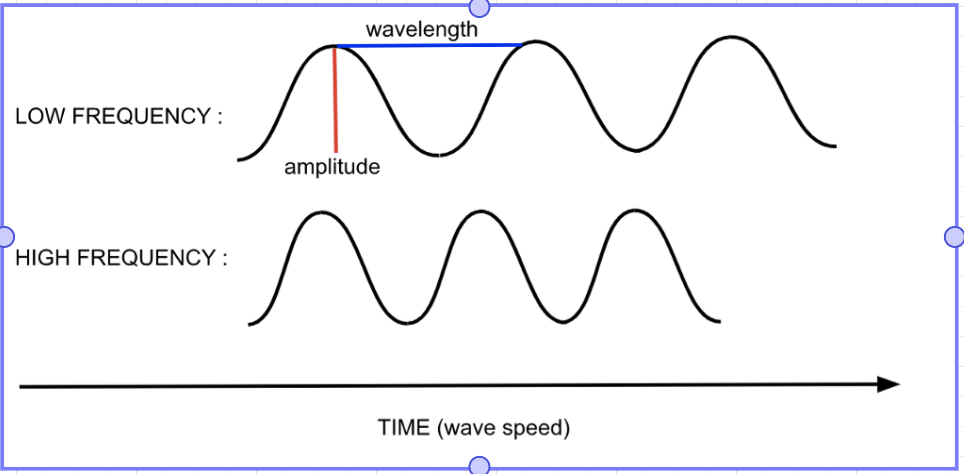
Long-wavelength = __ frequency
Short-wavelength = __ frequency
Wavelength and frequency of light are ______ to each other
long-wavelength = low frequency
short-wavelength = high frequency
inversely proportional
smaller mass = ___ waves
bigger mass = ___ waves
smaller mass = longer waves (more observable)
bigger mass = shorter waves (less observable)
Light =
Has properties of waves when _____
Has properties particles of when ____
form of electromagnetic radiation
moving through space (when energy is being transmitted)
interacting with matter (can only be absorbed by electrons in quanta)
Matterwaves =
wavelike behavior of particles
Visible light is (continuous/discrete)
Line spectrums/atomic emission spectrums are (continous/discrete)
continuous
discrete
Heisenberg’s uncertainty principal =
it’s impossible to know the position and moment of particles exactly
ground state =
n = 1
excited state =
when electron absorbs energy and moves up an energy level
The photoelectric effect =
(Define quanta)
a light-hitting metal experiment that proved that electrons can only absorb specific amounts of energy, aka, quanta, aka, photons of light
Conversions:
meter → kilometer
meter → millimeter
meter → micrometer
meter → nanometer
x 10^-3
x 10³
x ^10^6
x10^9
Units of:
Frequency
Waves
Energy
Hz
m
J
How to calculate energy based. on frequency
ex.). A photon has a frequency of 2.68 × 10^6 Hz. Calculate its energy
(#1 of Practice problems)
E = 1.78 × 10^-27 J
How to calculate frequency based on wavelength
ex.) Find the frequency of a green light that has a wavelength of 545 nm
5.50 × 10^14 Hz
Don’t forget units!
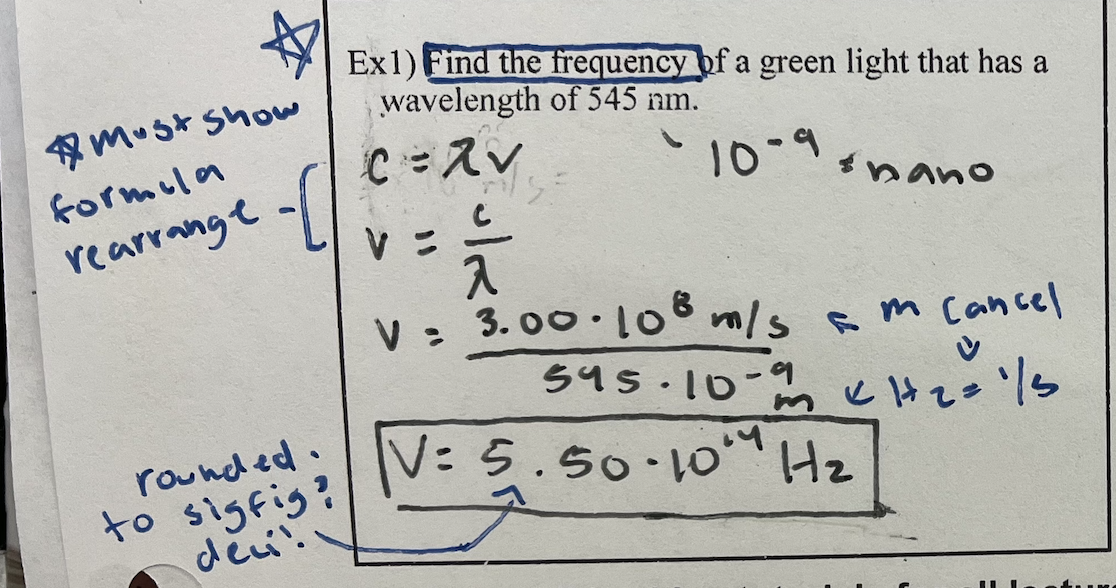
How to calculate wavelength and energy using frequency
ex.) Find the energy and wavelength of a photon of light with a frequency of 6.165 × 10^14 Hz
(#2 of Practice Problems)
E = 4.1 × 10^-19 J
Wavelength = 4.87 × 10^-7 m
don’t foget units!!
How to calculate number of photons
ex.) Calculate the. number of photons having a wavelength of 10.0 micrometers required to produce 1.0 kj of energy
(#6 of Practice problems)
5.0 × 10^ 22 photons
How to calculate energy per photon
ex.)
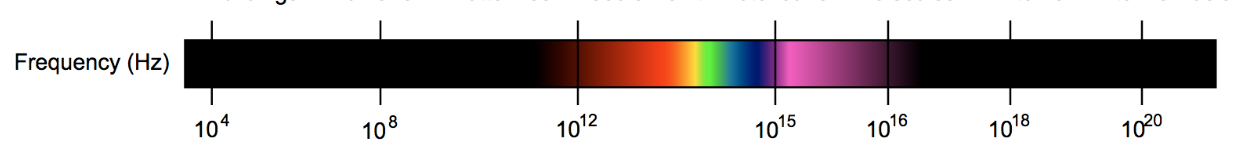
How to tell the type of electromagnetic radiation?
Frequency
Electron configuration of an atom =
distribution of electrons among orbitals
Explain how electrons move to higher and lower energy levels
an electron absorbs a certain quanta of energy → moves into a certain higher energy level
an electron loses certain quanta of energy and emits light → moves down energy levels
Principal energy level =
orbital where the electron is located (its sublevel)
Bohr model =
Quantum mechanic model/electron cloud model =
Electrons have orbits (clear, determined paths) dictated by quanta
Treats the election as a wave: Electrons have orbitals— where the electron is most likely to be based on its principal energy level— but can vary in shape by sublevels (s, p, d, etc)
Light emitted from an electron moving energy down energy levels is directly proportional to _______
the energy change of the electron
3 rules for elec configs.
Aufbau: Electrons occupy lowest levels first (Aufbau, like ‘A’ B C)
Pauli Exclusion:: Orbitals can hold at most 2 electrons, only if they spin in opposite directions (Pauli, like “pair”)
Hund’s: Single electrons fill up as many orbitals in a level as possible before doubling up
How to do elec configs? (not noble gas notation
ex) Find the electron configuration of KNO₃ (first compound of data page of L
Find your element on the table (K = Potassium)
Go through all the elements before your element and write down their orbital + amnt of elecs in that orbital, aka, how many elements in that group
a: 1s²+2s²+2p^6+3s²+3p^6+4s^1
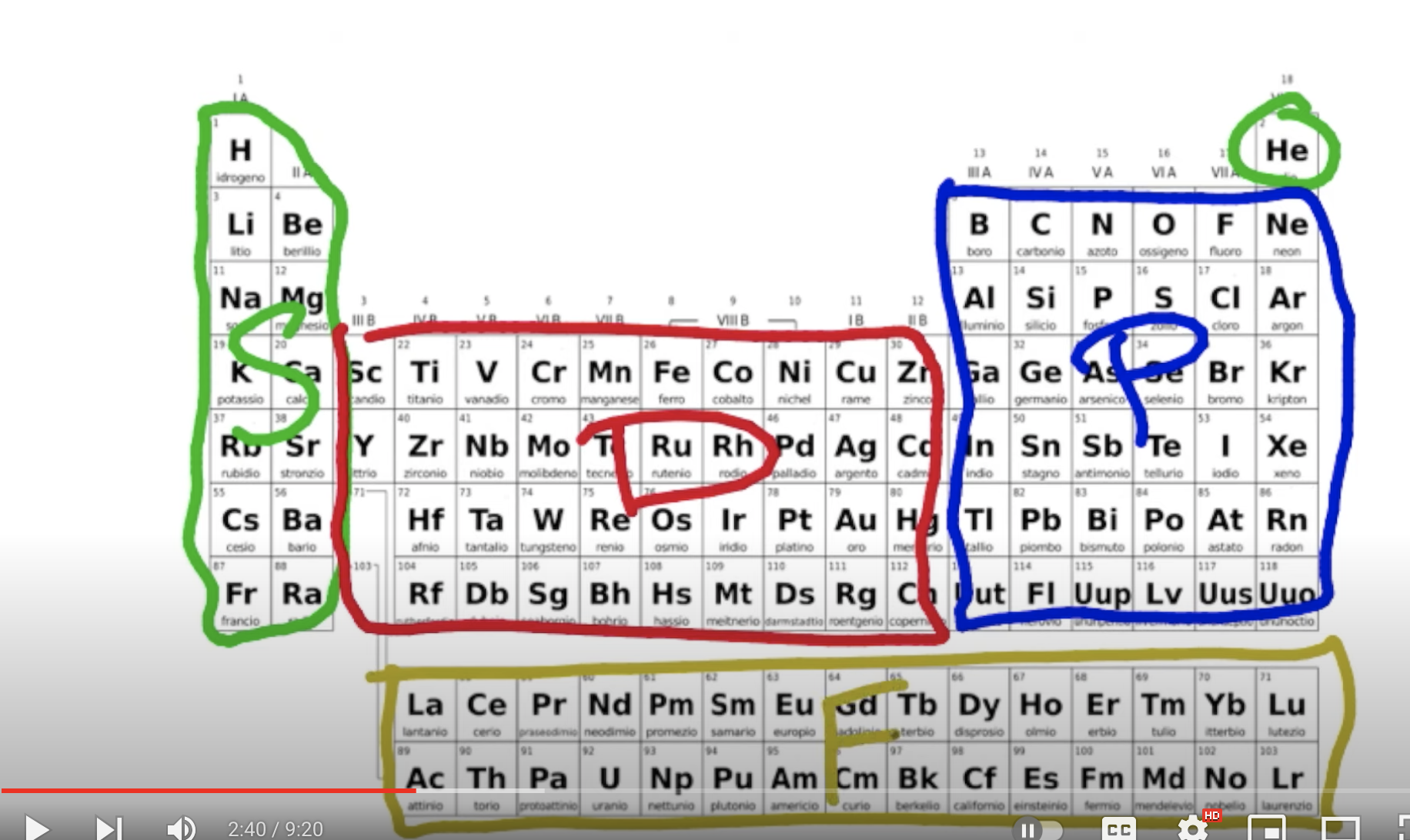
How to do noble gas notation?
ex) Find the electron configuration of KNO₃ (first compound of data page of L
Find your element on the table (K = Potassium)
Go up one row and all the way to column 18 to find your noble gas and box it [Ar]
After your noble gas, continue the configuration until you reach your element
continue config by adding each orbital for each element (K, after Ar, is s1) (you can know what sublevel is each element based on where it is on the periodic table)
a: [Ar] 4s1
![<ol><li><p>Find your element on the table (K = Potassium)</p></li><li><p>Go up one row and all the way to column 18 to find your noble gas and box it [Ar]</p></li><li><p>After your noble gas, continue the configuration until you reach your element</p><ul><li><p>continue config by adding each orbital for each element (K, after Ar, is s1) (you can know what sublevel is each element based on where it is on the periodic table)</p></li></ul></li></ol><p>a: <strong>[Ar] 4s1</strong></p>](https://knowt-user-attachments.s3.amazonaws.com/0f2cbbb4-f6b6-4749-a8a3-9f1a119db921.jpeg)