Atoms and Elements
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
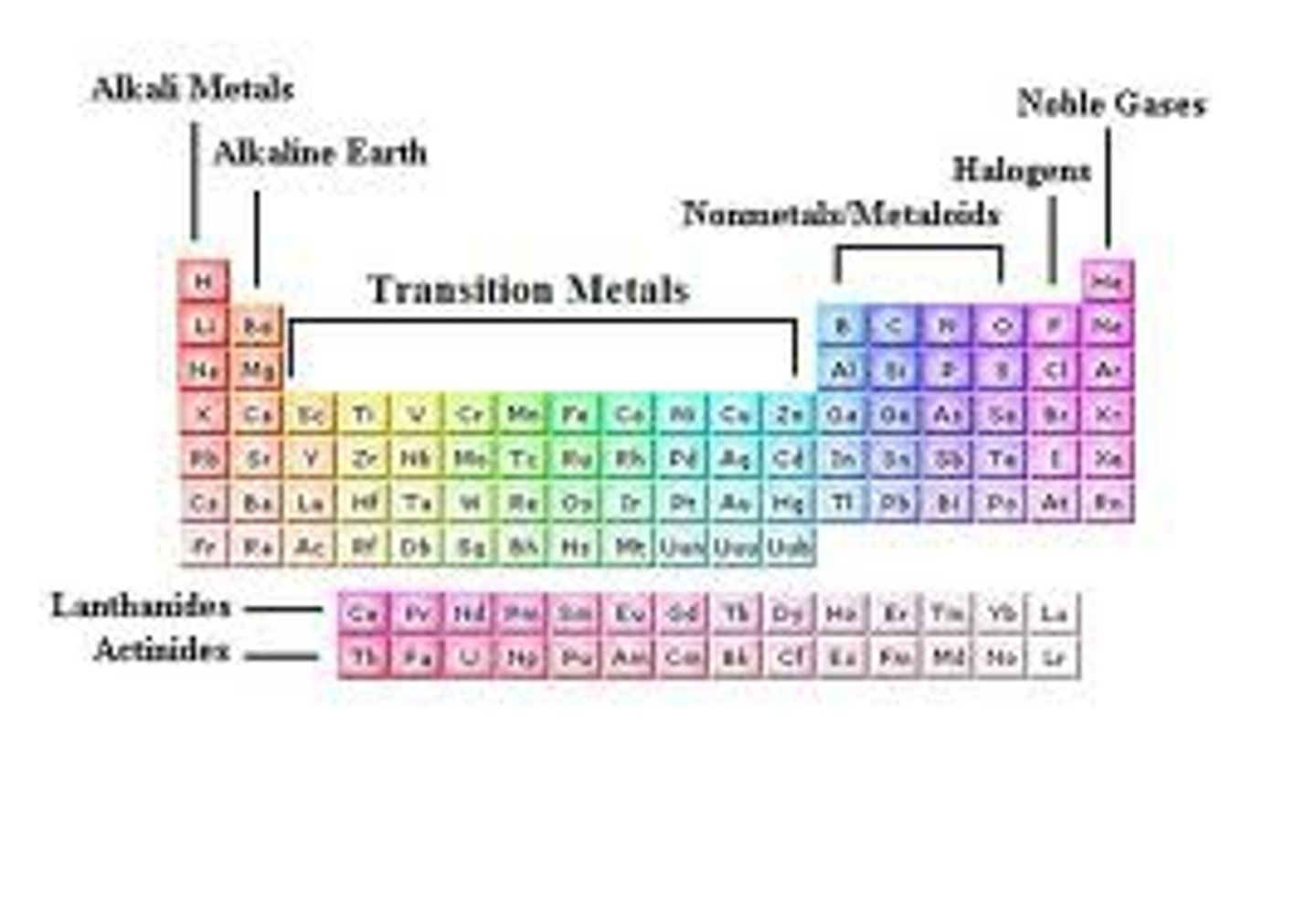
Periodic Table of Elements
Chart used to organize all known elements
Nucleus
the center of an atom, which contains the protons and neutrons
Element
A pure substance made of only one kind of atom
Atom
the smallest particle of an element that retains the properties of that element
Bohr Model
model of an atom that shows electrons in circular orbits around the nucleus
Symbol for aluminum
Al
Symbol for sodium
Na
Element used in balloons
Helium
Period
A horizontal row of elements in the periodic table
Group
A vertical column in the periodic table, also known as a family of elements
Proton
A subatomic particle that has a positive charge and that is found in the nucleus of an atom
Neutron
A subatomic particle that has no charge and that is found in the nucleus of an atom
Electron
A subatomic particle that has a negative charge
Atomic number
the number of protons in the nucleus of an atom; identifies the atom.
Element Symbol
the abbreviation of an elements name; 1 or 2 letters, first letter is always capitalized, second is never capitalized
Atomic mass
The decimal number on an element square; Number of protons and neutrons
Electron orbit
the path of an electron around the nucleus of an atom. Electrons are found here.
Subatomic particles
smaller particles that make up atoms
Compound
Two or more different elements joined by chemical bonds
18
The number of groups on the Periodic Table
7
The number of periods on the Periodic Table
metals
elements that are shiny, ductile, and good conductors of heat/electricity
noble gases
the elements in Group 18 on the periodic table
nonmetals
elements that are poor conductors of heat/electricity, dull, brittle
element square
the block on the periodic table that tells information about a specific element.
metalloids
Elements that have properties of both metals and nonmetals.
family
a group of elements on the periodic table that share similar properties
2
The number of electrons that can fit in the first shell
8
The number of electrons that can fit in the 2nd shell
atomic mass - atomic number
formula for calculating the number of neutrons
protons and neutrons
The nucleus of an atom contains
protons and electrons
Atomic number is equal to the number of...
Dmitri Mendeleev
Russian chemist who developed a periodic table of the chemical elements and predicted the discovery of several new elements