Chemistry: Week 7
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
At equilibrium, a solution contains [CH3CO2H] = 0.0787 M and [H3O+]=[CH3CO2−]=0.00118M. What is the value of Ka for acetic acid?
Substitution
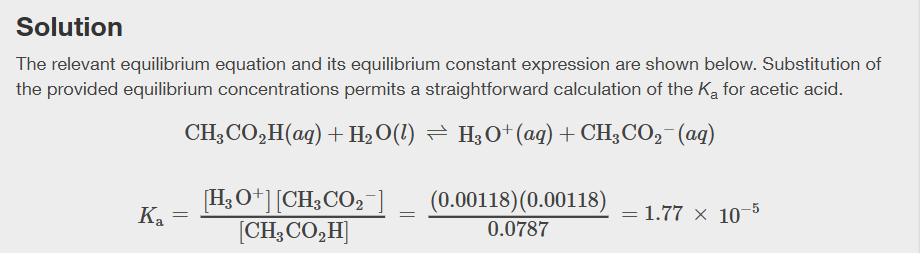
How does the acid strength of compounds of hydrogen with nonmetals go as H-A bond strength decreases down a group in the periodic table?
Acid strength of binary compounds of Hydrogen with nonmetals increases as H-A bond strength decreases down a group in the periodic table
What happens across a row in the periodic table to the acid strength of Hydrogen compounds?
Across a row, the acid strength of binary compounds increases with increasing electronegativity of the nonmetal atom, since polarity of H- A bond increases
Table for increasing acid strength and increasing base strength
Ternary Compounds
A chemical compound composed of three different elements
made of hydrogen, oxygen, and some third element “E”
compounds may be acidic, basic, or amphoteric depending on properties of E atom
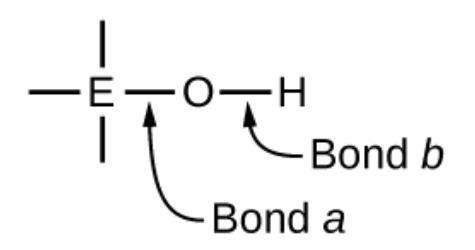
What happens if the central atom E has a low electronegativity in ternary acids and bases?
If the central atom, E, has a low electronegativity, its attraction for electrons is low
Central atom doesn’t form a strong covalent bond with the oxygen atom and the bond between the element and oxygen is more easily broken than bond between oxygen and hydrogen
Bond is ionic, hydroxide ions are released, and material is a base
What happens if the atom E has a high electronegativity in a ternary compound?
If E has a high electronegativity, it attracts the electrons it shares with oxygen and makes the bond covalent
B is weakened because electrons are displaced towards E
Bond b is polar and releases hydrogen ions to the solution, so material behaves as an acid