chapter 6 : shapes of molecules and intermolecular forces
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
how do lone pairs affect bond angles
lone pairs repel more strongly than bonded pairs so they push bonded pairs closer together which decreases the bond angle by approximately 2.5 degrees for each lone pair
what is an example and what are the bond angles and how many electron regions, bonding regions and lone pairs there for a linear shapethere
carbon dioxide
180 degrees
2
2
0
what is an example and what are the bond angles and how many electron regions, bonding regions and lone pairs there for a trigonometry planar shape
BF3
120 degrees
3
3
0
what is an example and what are the bond angles and how many electron regions, bonding regions and lone pairs there for a tetrahedral shape
CH4
109.5 degrees
4
4
0
what is an example and what are the bond angles and how many electron regions, bonding regions and lone pairs there for a pyramidal shape
NH3
107 degrees
3
3
1
what is an example and what are the bond angles and how many electron regions, bonding regions and lone pairs there for a bent / non-linear shape
H2O
104.5 degrees
4
2
2
what is an example and what are the bond angles and how many electron regions, bonding regions and lone pairs there for a triginal bipyramidal shape
PCL5
120 / 90 degrees
5
5
0
what is an example and what are the bond angles and how many electron regions, bonding regions and lone pairs there for an october deal shape
SF6
90 degrees
6
6
0
what is electronegativity
ability of an atom to attract electrons in a covalent bond
what 3 factors affect electronegativity
nuclear charge
shielding
atomic radius
how is electronegativity measured
using the pauling scale which compares elements to fluorine which is the most electronegative element
what three elements can have hydrogen bonds
fluorine oxygen and nitrogen
draw a hydrogen bond between two water molecules
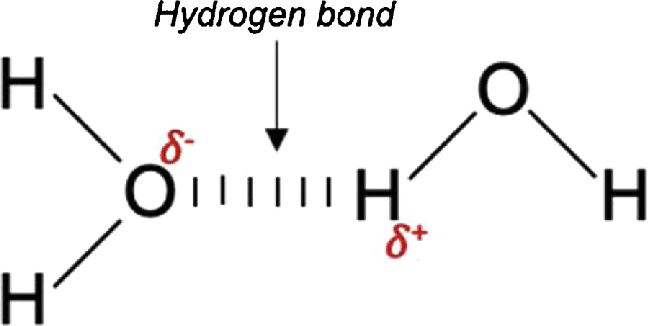
what are the 3 types of intermolecular forces from strongest to weakest
hydrogen bonding
permanent dipoles
london forces
how does electronegativity change as you go across the periodic table
it increases as nuclear charge increases, shielding stays the same and atomic radius decreases
how does electronegativity change as you go down the periodic table
it decreases as atomic charge increases, shielding increases and atomic radius increases. atomic radius and shielding have a greater affect on electronegativity than atomic charge does though
what are the 4 most electronegative elements
the non-metals oxygen fluorine nitrogen chlorine
what are the 3 least electronegative elements
group 1 metals lithium sodium and potassium
how do ionic bonds form
if electronegativity is very differdnt between two covalently bonded atoms, one bonded atom will have a greater attraction to the shared pair of electrons and will pull it away from the other atom till it’s no longer shared
what is a non-polar bond
a covalent bond where the electrons are shared equally between the bonded atoms
in what 2 cases there be a non-polar bond
if the two atoms are the same
if the two atoms have the same or similar electronegativity
what is a polar covalent bond
a covalent bond where the elements have different electronegativity values
what is a dipole
a difference in charge between two atoms caused by a shift in electron density in a bond causing a separation in opposite charges
what is a permanent dipole
a dipole that doesn’t change
what is the relative repulsion of different types of bonds from weakest to strongest
bonded pair - bonded pair, bonded pair - lone pair, lone pair - lone pair
what is an intermolecular force
weak interactions between dipoles of different molecules
what are london forces
weak induced dipole-dipole bonds between all molecules whether polar or non-polar
how are induced dipoles formed (4)
the movement of electrons produce a changing dipole
an instantaneous dipole will exist (though it is constantly changing)
the instantaneous dipole will induce a dipole in another molecule
the induced dipole induces further dipoles in other molecules which then attract one another
what are 3 effects of more electrons in a molecule on london forces
larger instantaneous and induced dipoles
stronger attractive forces between molecules
greater induced dipole-dipole interactions
how does the number of electrons affect boiling point
the more electrons, the stronger the london forces so more energy is required to break the bonds to change state which means a greater boiling point
what is a simple molecular substance
simple molecules of small units containing a definite number of atoms with a definite molecular formula
what structure do simple molecular substances form as a solid
simple molecular lattices
what are two features of simple molecular lattices
molecules are held in place with weak intermolecular forces
atoms within each molecule are held together strongly by covalent bonds
what are the melting and boiling points like for simple molecular substances
low
what happens when simple molecular substances melt
weak intermolecular forces break but strong covalent bonds don’t
what happens when a simple molecular compound is added to a non-polar solvent like hexane
intermolecular forces form between the substance and solvent which weakens intermolecular forces in the simple lattice causing it to break apart and the substance to dissolve
are simple molecular substances soluble or insoluble in polar solvents and why
they are insoluble because the intermolecular forces (dipole-dipole bonds) in the polar solvents are too strong to break to dissolve
why are simple molecular substances not conductors
because there’s no charged particles that can move
why can polar substances sometimes dissolve in polar solvents
because the polar substance molecules and polar solvent molecules can attract each other and break the lattice apart
what is a hydrogen bond
a type of dipole-dipole bond between a molecule with an electronegative atom with a lone pair and a molecule with a hydrogen atom attached to an electronegative atom
what are 3 examples of electronegative atoms within each molecule a lone pair of electrons
oxygen nitrogen fluorine
how do you represent a hydrogen bond
a dashed line
why is solid ice less dense than liquid water
hydrogen bonds hold water molecules apart in an open lattice structure so water molecules are further apart in solids than liquids meaning ice is less dense
why does water have a relatively high melting and boiling point
they have hydrogen bonds as well as london forces so more energy is required to break these extra bonds which means a higher melting and boiling point as the lattice in ice needs to be broken for it to melt