transition metals
1/67
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
68 Terms
review exceptions for
chromium and copper electron configs
period 4 and 5 general atomic configs
[noble gas]ns²(n-1)dx
period 6 and 7 (ha) general atomic configs
[noble gas]ns²(n-2)f14(n-1)dx
when removing electrons, transition metals lose
s electrons before d electrons
tm cations with same configs have
similar properties
across a period, size of TM metals
decreases, but less than main group because the many d electrons in the inner energy level shield outer s electrons
down a group, size of TM metals
does not change significantly; adding an extra shell increases size but lanthanide contraction shrinks it
lanthanide contraction
extra protons cause a shrinkage
for period 4 transition elements, ionization energy
increases; not regular trend for period 5/6
between period 5 and 6,
ionization energy is higher because of a higher Zeff due to lanthanide contraction
oxidation number
charge that the atom would have if the electrons were transferred completely to/from bonded atoms
oxidation number for an atom in its elemental form
0
ON for a monoatomic ion
ion charge (sign before numeral)
sum of ON values of atoms in a molecule
0
sum of ON values of atoms in a polyatomic ion
charge of the ion
ON for groups 1 and 2 almost always
+1 and +2
ON for hydrogen
+1 when combined with nonmetals, -1 when combined with metals or boron
ON for oxygen
-2 in most cases
ON for group 17
-1 in most cases
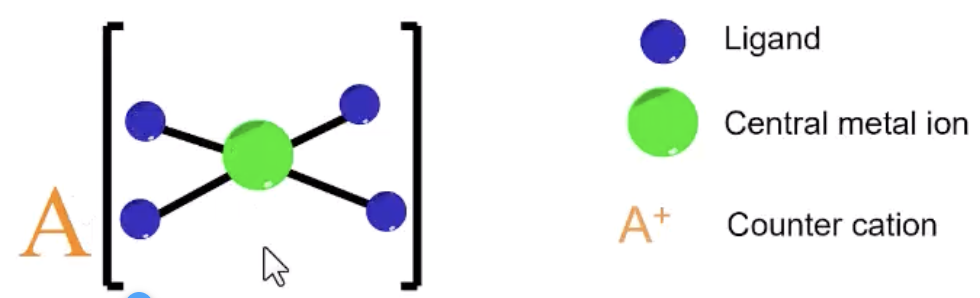
coordination compound
behave like covalent polyatomic ions in solution (ligands remain attached)
coordination compound ions associate with
counterions to achieve neutrality
coordination compounds written inside
square brackets
coordination compound structure
transition metal center with neutral or anionic ligands
ligand
molecule or anion with one or more donor atoms, each donating a pair of electrons to the metal ion to form a coordinate bond
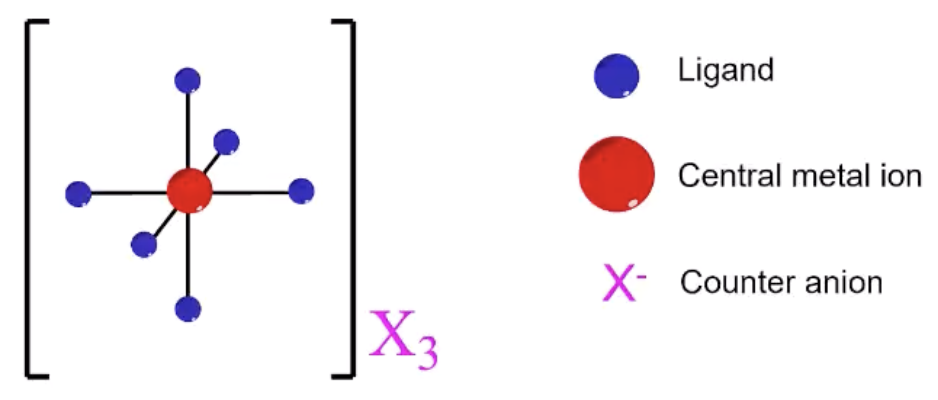
coordination compound illustrations
monodentate
only one bonding site
bi/polydentate
two/multiple bonding sites
coordination number
how many binding sites there are on the central metal ion; helps us figure out geometry
coordination number 2
linear geometry
coordination number 4 + d8 config on central metal ion
square planar
coordination number 4 + d10 or sometimes d5 on central metal ion
tetrahedral
coordination number 6
octahedral geometry
coordination isomer
same compound formula but different composition of the complex ion e.g. switching around ligand and counter ion
linkage isomer
same composition of the complex but the ligand donor atom is different; clue is if there is a ligand with more than one different type of atom that had a lone pair
isomerism of square planar
geometric (cis and trans)
isomerism of tetrahedral
4 different monodentate ligands can be chiral → enantiomers
naming a coordination compound
in brackets metal first, ligands next, counter ions go outside; cations first, anions second
NH3
ammine
H2O
aqua
NO
nitrosyl
en
ethylene diamine
CO
carbonyl
Cl-/I-/F-/Br-
chloro/iodo/fluoro/bromo
NO2-
nitro/nitrito
CN-
cyano
OH-
hydroxo
C2O42-
oxalato
number of each type of ligand indicated by
greek prefixes i.e. di, tri, tetra
if ligand already contains a greek prefix or if it is bi/polydentate then use
bis, tris, tetrakis, pentakis
ligands named in order of
alphabet, ignoring the greek prefix
roman numeral denotes
oxidation number of the central metal ion
suffix -ate is added to the metal’s name if
the complex has an overall negative charge
cation is written
before the anion
crystal field theory
as ligands begin to align themselves, the d orbitals do not remain degenerate (having the same energy)
crystal field effect
the splitting of orbitals, creating an energy difference between them, denoted as ∆
eg orbitals
those closest to ligands, so repel the most and have higher energy
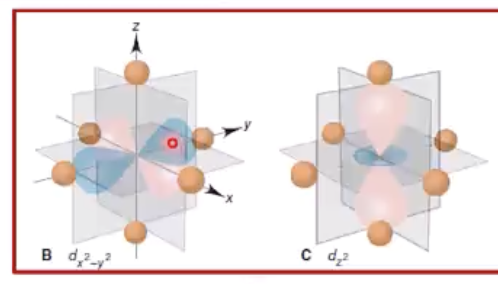
t2g orbitals
not close to ligands, have lower energy
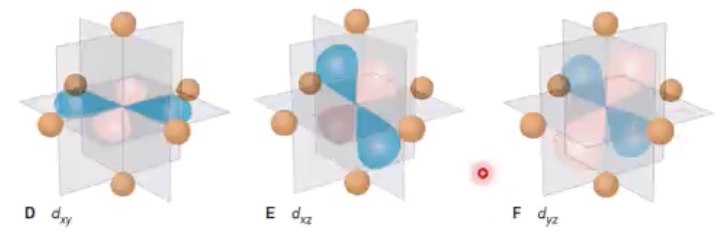
crystal field theory and color
electrons get excited from t2g to eg and reflects light

energy gap of the same metal depends on
ligands
strong field ligands
more electron density - more repulsion of the particular d orbitals, causing a larger difference in energy of t2g and eg, so a higher ∆
high energy in a strong field ligand →
low wavelength i.e. absorbing blue light
lower energy in a weak field ligand →
high wavelength i.e. absorbing red light
crystal field splitting energy
the energy difference between t2g and eg
spectrochemical series of ligands in order of increasing field strength
I-, Cl-, F-, HO-, H2O, SCN-, NH3, en, NO2-, NC-, CO
from d1 to d3, magnetic properties are
nonexistent because same number of unpaired electrons for strong or weak field
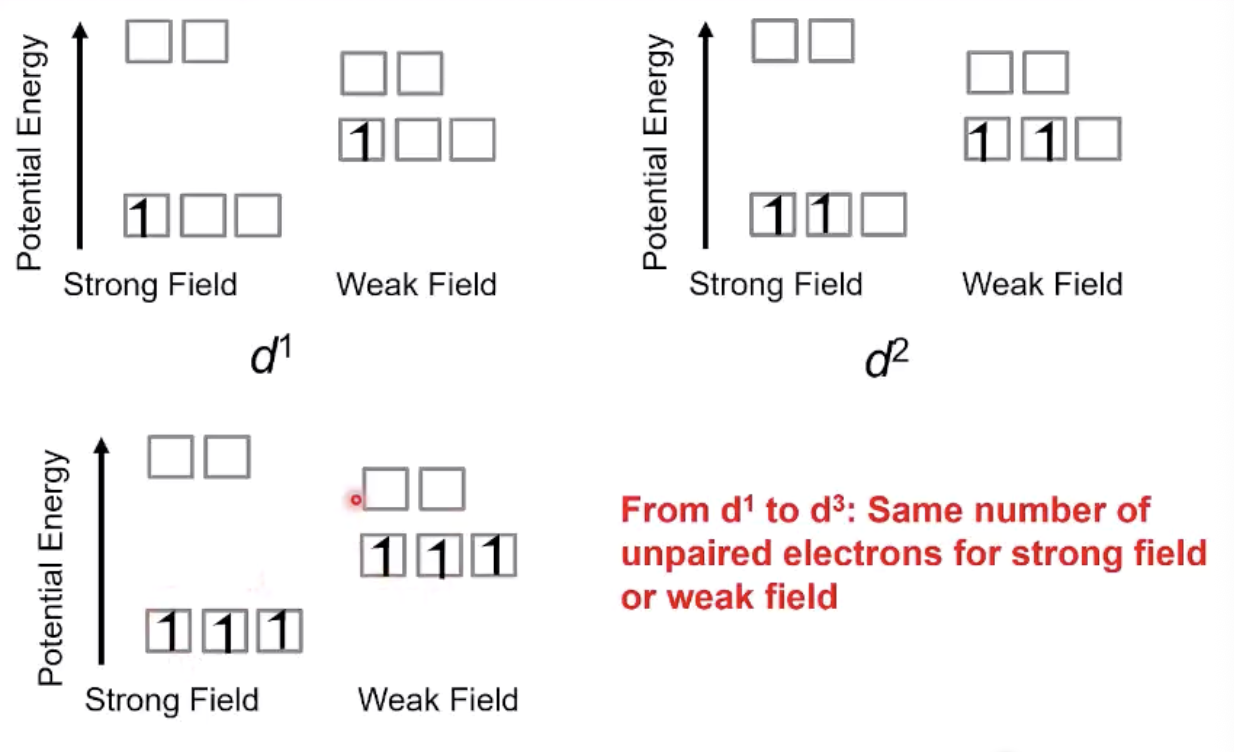
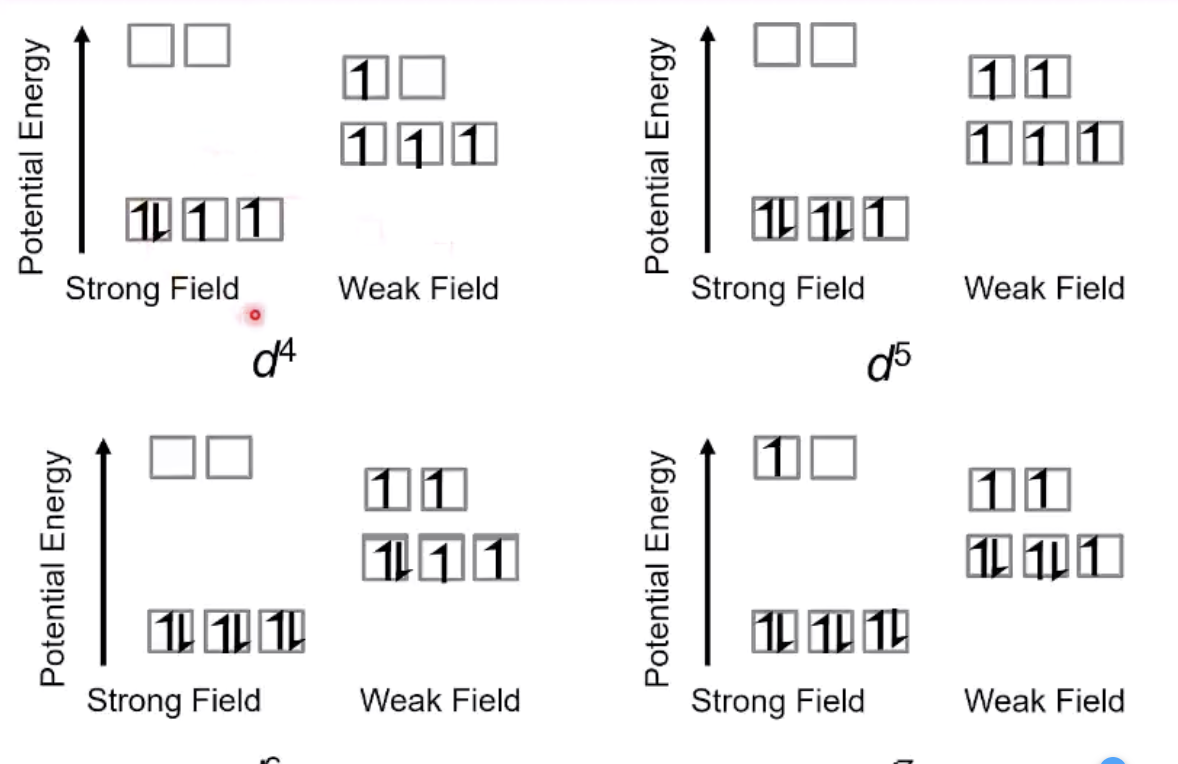
from d4 to d7
different properties because the excitation is different
oxidation number NH3
0
oxidation number NO2
0