Physics - Particles and Quantum Phenomena
1/68
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
69 Terms
What is a Nucleon?
It is a particle inside the nucleus of an atom, such as a proton or a neutron.
What are the masses and charges of a neutron, proton and electron?
Proton:
Charge (C) - +1.60×10-19 relative charge is 1
Mass (kg) - 1.67×10-27 relative mass is 1
Neutron:
Charge (C) - 0 relative charge is 0
Mass (kg) - 1.67×10-27 relative mass is 1
Electron:
Charge (C) - -1.60×10-19 relative charge is -1
Mass (kg) - 9.11×10-31 relative mass is 0.0005
What is a proton number?
Represented by Z is the atomic number of the atom.
What is an isotope?
Same number of protons but different number of neutrons.
What is a nucleon number?
The total number of protons and neutrons in an atom, sometimes called the mass number.
What is each type of nucleus called?
Nuclide
What is specific charge?
The specific charge of a charged particle is defined as its charge divided by its mass.
What is the strong nuclear force?
It overcomes the electrostatic force of repulsion between the protons in the nucleus and keeps the protons and neutrons together.
Its range is no more than 3-4 fm
It has the same effect between two protons as it does between two neutrons or a proton and a neutron.
It has an attractive force from 3-4 fm down to about 0.5 fm. At separations smaller than this, it is a repulsive force that acts to prevent neutrons and protons being pushed into each other.
What is alpha radiation?
Alpha particles which consist of two protons and two neutrons.
The symbol for an alpha particle is 42α.
AZX → A-4Z-2Y + ⁴₂He
What is beta radiation?
Symbol for an electron as a beta particle is β⁻ or 0-1β
It happens as a result of a neutron in the nucleus changing into a proton.
ⁿₐX → ⁿₐ₊₁Y + ⁰₋₁e + ν̄ₑ
What is gamma radiation?
Symbol is γ
It is electromagnetic radiation emitted by an unstable nucleus. It can pass through thick metal plates.
It has no mass and no charge.
It is emitted by a nucleus with too much energy, following an alpha or beta emission.
Why was the existence of the antineutrino hypothesised?
To account for conservation of energy in beta decay.
What is the equation linking wavelength, wave speed and wave frequency?
v = fλ
Where:
v = wave speed (m s⁻¹)
f = frequency (Hz)
λ = wavelength (m)
What does the electromagnetic spectrum consist of?
An electromagnetic wave consists of an electric wave and a magnetic wave which travel together and vibrate
at right angle to each other and to the direction in which they are travelling.
in phase with each other. The two waves reach a peak together so they are inn step. When waves do this they are in phase.
When are electromagnetic waves emitted?
When a fast moving electron is stopped or slows down or changes direction
An electron in a shell of an atom moves to a different shell of lower energy.
Electromagnetic waves are emitted as short bursts of waves, each burst leaving the source in a different direction. Each burst is a packet of electromagnetic waves and is referred to as a photon.
What is the equation linking photon energy to planks length to frequency?
Photon energy E=hf = (hc)/λ
h = Plank constant 6.63×10-34Js.
For a beam consisting of photons of frequency f
the power of the beam =nhf
What is antimatter?
For every type of particle there is a corresponding antiparticle that
Annihilates the particle and itself if they meet, converting their total mass into photons.
Has exactly the same rest mass as the particle
Has exactly opposite charge to the particle if the particle has a charge.
What is an electronvolt?
1 MeV = 1.60×10-13J
One electron volt is defined as the energy transferred when an electron is moved through a potential difference of 1 volt.
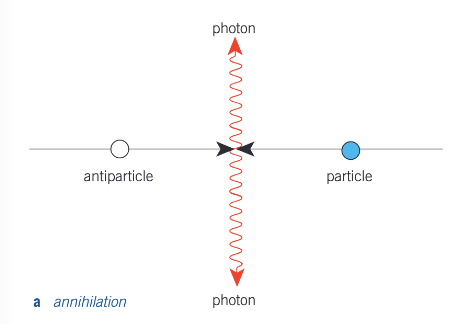
What is annihilation?
It occurs when a particle and a corresponding antiparticle meet and their mass is converted into radiation energy. Two photons are produced in this process (as a single photon cannot ensure a total momentum of zero after the collision). Therefore, the minimum energy of each photon, hfmin, is given by equation the energy of the two photons to the rest energy of the particle and of the antiparticle.
hfmin = E0
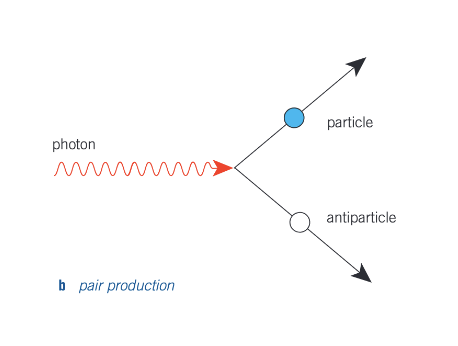
What is pair production?
A photon creates a particle and a corresponding antiparticle, and vanishes in the process.
Minimum energy of each photon produced, hfmin = 2E0
What are the corresponding antiparticles for electrons, protons, neutron and neutrinos?
Positron, antiproton, antineutron, antineutrino
What are the four fundamental interaction in the universe?
Gravity
Electromagnetic
Weak nuclear
Strong nuclear
How are forces exchanged?
The exchanging of particles explains the exchanging of forces. Exchanging particles also exchanges momentum.
What is the weak nuclear force?
Causes β⁻ and + decay.
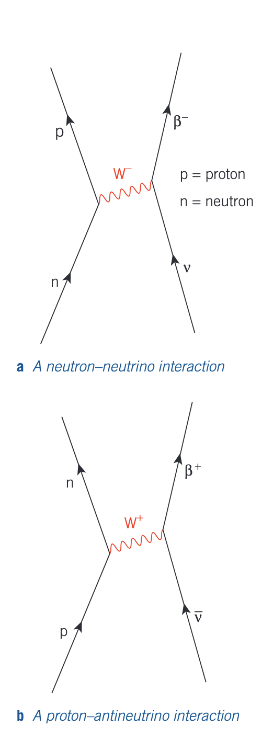
Give examples of when neutrinos and antineutrinos interact with each other. And state what causes them.
A neutrino can interact with a neutron and make it change into a proton. A β⁻ particle is created and emitted as a result of the change
An antineutrino can interact with a proton and make it change into a neutron. A β+ particle is created an emitted as a result of the change.
These interactions are due to the exchange of particles referred to as W bosons. These exchange particles:
have a non-zero rest mass
have a very short range of no more than about 0.001fm
are positively charged W+ boson or negatively charged W- boson.
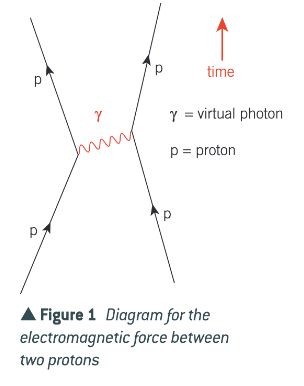
What causes the electromagnetic force?
The electromagnetic force between two charged objects is due to the exchange of virtual photons.
Beta decay - into electron
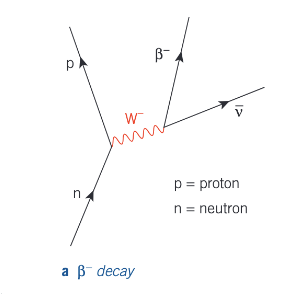
Beta decay - into positron
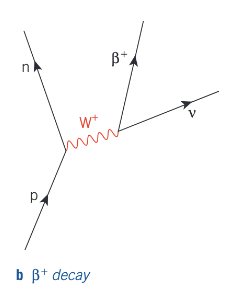
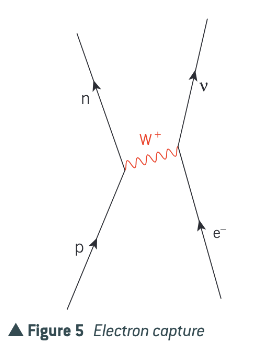
What is electron capture?
Sometimes a proton in a proton-rich nucleus turns into a neutron as a result of interacting through the weak interaction with an inner-shell electron from outside the nucleus (electron capture).
The same change can happen when a proton and an electron collide at very high speed. In addition, for an electron with sufficient energy, the overall change could also occur as a W- exchange from the electron to the proton.
State the charge, antiparticle and symbol, charge rest energy and interaction of the proton, neutron, electron, neutrino, muon, pion and kaon.
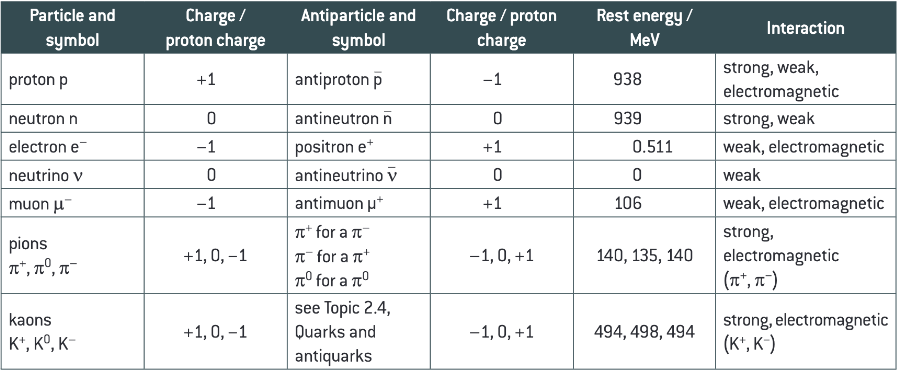
What are hadrons?
Particles and antiparticles that can interact through the strong interaction (e.g. protons, neutrons, π mesons, K mesons).
Hadrons can interact through all four fundamental interactions. They interact through the strong interaction and through the weak interaction and through the electromagnetic interaction if charged. Apart from the proton, which is stable, hadrons tend to decay through the weak interaction.
What are leptons?
Particles and antiparticles that do not interact through the strong interaction (e.g. electrons, muons, neutrinos).
Leptons interaction through the weak interaction, the gravitational interaction, and through the electromagnetic interaction (if charged).
What are baryons?
Are protons and all other hadrons (including neutrons) that decay into protons, either directly or indirectly.
What are mesons?
Are hadrons that do not include protons in their decay products. In other words, kaons and pions are not baryons.
What are baryons and mesons made up of?
Quarks and antiquarks.
What is the symbol for a muon neutrino?
Muon neutrino: νₘᵤ (often written as ν_μ)
Muon antineutrino: ν̄ₘᵤ (often written as ν̄_μ)
What is the symbol for the electron neutrino?
νₑ
ν̄ₑ
What are the different lepton rules?
In an interaction between a lepton and a hadron, a neutrino or antineutrino can change into or form a corresponding charged lepton.
In muon decay, the muon changes into a muon neutrino. In addition, an electron is created to conserve charge and a corresponding antineutrino is created to conserve lepton number.
The lepton number is conserved in any change.
The lepton number is +1 for any lepton, -1 for any antilepton and 0 for any non-lepton.
The lepton number is a quantum number that does not change.
What can a kaon and a sigma particle decay into?
A kaon decays into pions
A sigma particle:
Has a different rest mass to the kaon, which was always greater than the rest mass of the proton’s rest mass.
Decay either in sequence or directly into protons and pions.
What is strangeness?
All the observed reactions conserve charge. To explain why certain reaction were not observed, a strangeness number S was introduced for each particle and antiparticle (starting with +1 for the K+ meson) so that strangeness is always conserved in strong interactions. Non-strange particles (i.e. the proton, neutron, pions, leptons) were assigned zero strangeness. The strangeness numbers for the other strange particle and antiparticle can then be deduced from the observed reactions.
Strangeness is always conserved in a strong interaction, whereas strangeness can change by 0, +1 or -1 in weak interactions.
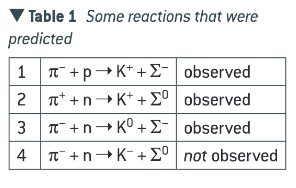
How are strange particles created and decay?
Produced through the strong interaction and decay through the weak interaction.
What is strangeness?
Strangeness is a quantum number which reflects the fact that strange particle are always created in pairs.
What are the different quarks and their properties? And the different quark combinations for the mesons?
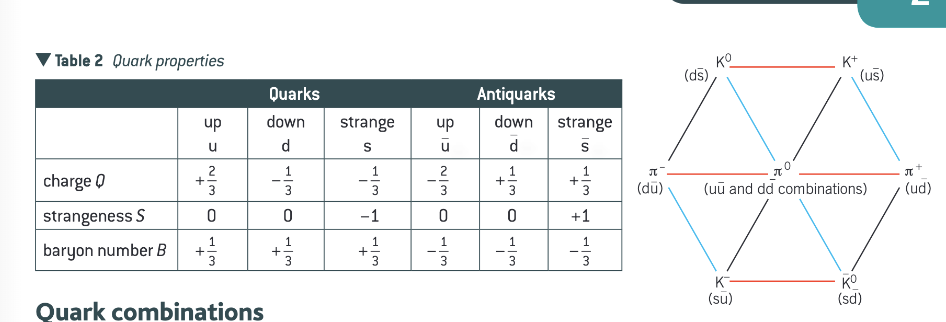
What are baryons and antibaryons?
Hadrons that consist of three quarks for a baryon or three antiquarks for an antibaryon.
A proton is the uud combination
A neutron is the udd combination.
An antiproton is the anti(uud) combination
The Σ particle is a baryon containing a strange quark.
The proton is the only stable baryon. A free neutron decays into a proton, releasing an electron and an electron antineutrino , as in β⁻ decay.
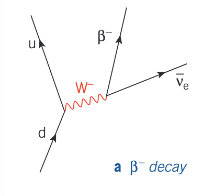
How does quark character change in β⁻ decay?
In β⁻ decay, a neutron in a neutron-rich nucleus changes into a proton, releasing an electron and an electron antineutrino. In quark terms, a down quark changes to an up quark.
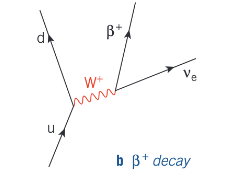
How does quark character change in β+ decay?
In β+ decay, a proton in a proton-rich nucleus changes into a neutron, releasing a positron and an electron neutrino. In quark terms, an up quark changes to a down quark.
What are the different conservation rules that particles obey?
Conservation of energy and conservation of charge.
Conservation of lepton numbers.
Conservation of strangeness
Conservation of baryon number - in any reaction, the total baryon number is conserved.
What does the pion do?
It is the exchange particle of the strong nuclear force.
What does a muon decay into?
It decays into an electron
What does particle physics rely on in terms of development?
Collaborative efforts of large teams scientists and engineers to validate new knowledge.
What is the photoelectric effect?
The effect of electromagnetic radiation on metals showed that electrons are emitted from the surface of a metal when electromagnetic radiation above a certain frequency was directed at the metal.
Photoelectric emission of electrons from a metal surface does not take place if the frequency of the incident electromagnetic radiation is below a certain value known as the threshold frequency. This minimum frequency depends on the type of metal. This means that the wavelength of the incident light must be less than a maximum value equal to the speed of light divided by the threshold frequency.
The number of electrons emitted per second is proportional to the intensity of the incident radiation.
Photoelectric emission occurs without delay as soon as the incident radiation is directed at the surface, provided the frequency of the radiation exceeds the threshold frequency, and regardless of intensity.
What did Einstein suggest in order to explain the photoelectric effect?
When light is incident on a metal surface, an electron at the surface absorbs a single photon from the incident light and therefore gains energy equal to hf, where hf is the energy of a light photon.
An electron can leave the metal surface if the energy gained from a single photon exceeds the work function ϕ of the metal. This is the minimum energy needed by an electron to escape from the metal surface.
Excess energy gained by the photoelectron becomes its kinetic energy.
What is the equation linking maximum kinetic energy to planks costant, frequency and the work function?
hf = EKmax + ϕ
What is EKmax?
The maximum kinetic energy of the photoelectrons.
What is stopping potential?
The minimum potential needed to stop photoelectric emission.
At this potential, the maximum kinetic energy of the emitted electron is reduced to zero because each emitted electron must do work equal to e x Vs to leave the metal surface. Hence its maximum kinetic energy is equal to e x Vs.
What is ionisation?
The process of creating an ion.
What is an electron volt?
The electron volt is a unit of energy equal to the work done when an electron is moved through a pd of 1V = 1.6×10-19C.
What is excitation?
When atoms abosrb energy from colliding electrons without being ionised.
What are excitation energies?
The energy value at which an atom absorbs energy are known as its excitation energies.
What are the different energy states of an electron?
Ground state - when the electron is in the lowest energy state of an atom.
Excited state - when an atom in the ground state absorbs energy, on of its electrons moves to a shell at higher energy, so the atom is now in an excited state.
Energy levels - Electrons take different energy levels.
What is de-excitation?
When an electron moves to a lower energy level and emits a photon, where the energy of the photon is equal to the energy lost by the electron and therefore by the atom.
What is the general equation for de-excitation?
In general, when an electron moves from energy level E1 to a lower energy level E2
The energy of the emitted photon hf = E1 - E2
Why does fluorescence occur?
An atom in an excited state can de-excite directly or indirectly to the ground state, regardless of how the excitation took place. An atom can absorb photons of certain energies and then emit photons of the same or lesser energies.
This overall process why certain substances fluoresce or glow with visible light when they absorb ultraviolet radiation.
How does fluorescence take place in a fluorescent tube?
A fluorescent tube is a glass tube with a fluorescent coating on its inner surface. The tube contains mercury vapour at low pressure. When the tube is on, it emits visible light because:
Ionisation and excitation of the mercury atoms occur as they collide with each other and with electron in the tube
the mercury atoms emit ultraviolet photons, as well as visible photons and photons of much less energy, when they de-excite.
the ultraviolet photons are absorbed by the atoms of the fluorescent coating, causing excitation of the atoms
the coating atoms de-excite in steps and emit visible photons.
How can light spectra be evidence for transitions between discrete energy levels in atoms?
If we use a tube of glowing gas as the light source, we see a spectrum of discrete lines of different colours.
The wavelengths of the lines of a line spectrum of an element are characteristic of the atoms of that element. By measuring the wavelengths of a line spectrum, we can therefore identify the element that produced the light. No other element produces the same pattern of light wavelengths. This is because the energy levels of each type of atom are unique to that atom.
An atom emits the photon when an electron de-excites.
What are the dual natures of light?
The wave-like nature is observed when diffraction of light takes place. This happens, for example, when light passes through a narrow slit. The light emerging from the slit spreads out in the same way as water waves spread out after passing through a gap. The narrower the gap or the longer the wavelength, the greater the amount of diffraction.
The particle-like nature is observed, for example, in the photoelectric effect. When light is directed at a metal surface and an electron at the surface absorbs a photon of frequency f, the kinetic energy of the electron is increased from a negligible value by hf. The electron can escape if the energy it gains from a photon exceeds the work function of the metal.
What is the de Broglie hypothesis?
That matter also has a particle and wave nature/ dual nature.
matter particles have a dual wave-particle nature
the wave-like behaviour of matter particle is characterised by a wavelength, its de Broglie wavelength, λ, which is related to the momentum, p, of the particle by means of the equation λ = h / p or λ = h / (mv)
What evidence is there for de Broglie’s hypothesis?
A narrow beam of electrons in a vacuum tube is directed at a thin metal foil. A metal is composed of many tiny crystalline regions. Each region, or grain, consists of positive ions arranged in fixed positions in rows in a regular pattern. The rows of atoms cause the electrons in the beam to be diffracted, just as a beam of light is diffracted when it passes through a slit.
The electrons in the beam pass through the metal foil and are diffracted in certain directions only. They form a pattern of rings on a fluorescent screen at the end of the tube. Each ring is due to electrons diffracted by the same amount from grains of different orientations, at the same angle to the incident beam.
The beam of electrons is produced by attracting electrons from a heated filament wire to a positively charged metal plate, which has a small hole at its centre. Electrons that pass through the hole form the beam. The speed of these electrons can be increased by increasing the potential difference between the filament and the metal plate. This makes the diffraction rings smaller, because the increase of speed makes the de Broglie wavelength smaller. So less diffraction occurs and the rings become smaller.
How does knowledge and information of the nature of matter change and how is it evaluated?
The knowledge of the nature of matter changes over time.
Such changes need to be evaluated through peer review and validated by the scientific community.