Intermolecular Forces
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
Dipole-dipole forces
Partial charges interact with other partial charges on other polar molecules. These interactions are small and weak, but get stronger with an increasing number of interacting molecules
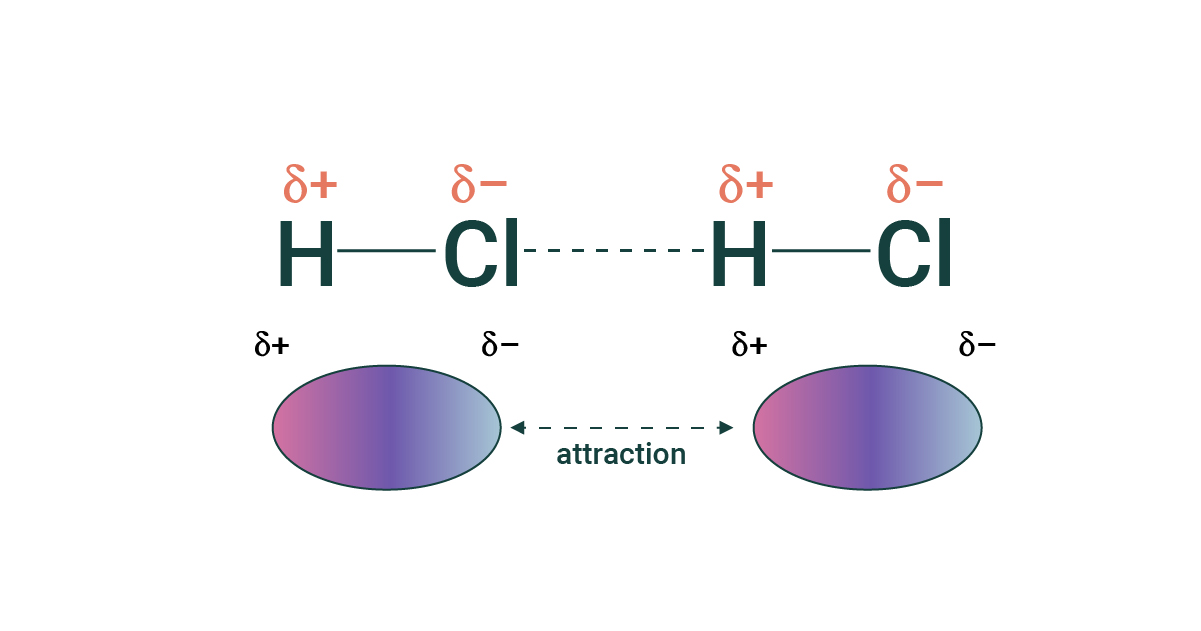
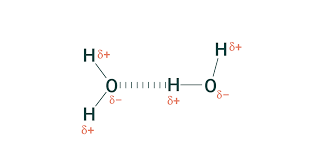
Hydrogen bonding
A type of dipole-dipole interaction, but with hydrogen. Generally occurs between O-H and N-H. Interaction energy is stronger than dipole-dipole
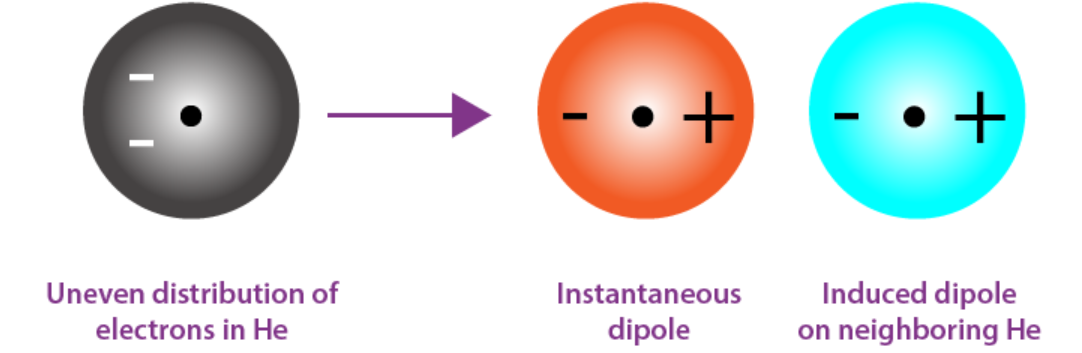
London dispersion forces
Weakest forces caused by temporary dipoles. The more linear the molecules are, the more attracted they will be to each other. Energy is also greater for molecules with higher molecular weight (# of electrons)
Polarizability
How tightly held electrons are to the nucleus of an atom