Inorganic Chemistry Exam 1
1/35
Earn XP
Description and Tags
Units 1-4
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
Name the molecular geometry for:
3 electron pairs and no lone pairs
Trigonal Planar
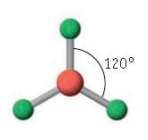
Name the molecular geometry for:
4 electron pairs and no lone pairs
tetrahedral
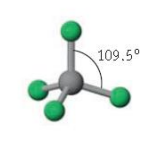
Name the molecular geometry for:
5 electron pairs no lone pairs
trigonal bipyramidal
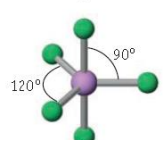
Name the molecular geometry for:
6 electron pairs and no lone pairs
octahedral
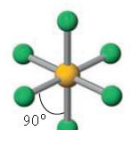
Name the molecular geometry for:
2 electron pairs and 1 lone pair
Bent
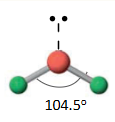
Name the molecular geometry for:
3 electron pairs and one lone pair
trigonal bipyramidal
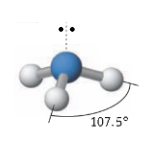
Name the molecular geometry for:
4 electron pairs and one lone pair
seesaw
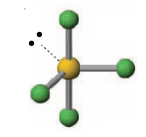
Name the molecular geometry for:
5 electron pairs and one lone pair
square pyramidal
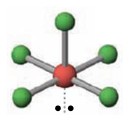
Name the molecular geometry for:
2 electron pairs and 2 lone pairs
T-shaped
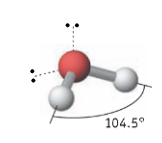
Name the molecular geometry for:
3 electron pairs 2 lone pairs
T-shaped
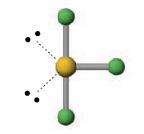
Name the molecular geometry for:
4 electron pairs 2 lone pairs
square planar
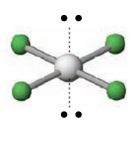
Name the molecular geometry for:
2 electron pairs and 3 lone pairs
Linear
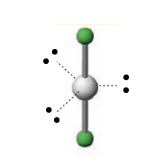
Linear bond angle
180
Trigonal planar bond angle
120
Tetrahedral bond angle
109.5
Trigonal bipyramidal bond angle
120 and 90
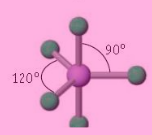
Octahedral bond angle
90
As the number of lone pairs increase, the angle between other bonds ____________
decreases
True or False: Double and Triple bonds are MORE repulsive than single bonds.
True
Rule for L quantum number
n - 1
n represents
principal quantum number
L quantum number represents
angular distribution or shape of the orbital
The quantum number mL represents
magnetic quantum number or orientation of orbitals within a subshell
Rules for mL quantum number
-L to +L
Quantum number ms represents
Electron spin
Principal shell with n = 1 has __ subshell L=
one s, L=0
Principal shell with n = 2 has __ subshell. L=
one s and one p, L=0,1
Principal shell with n = 3 has __ subshell
one s subshell, one p subshell, and one d, L= 0, 1, 2
Number of orbitals in an S subshell
1
Number of orbitals in a P subshell
3
Number of orbitals in a D subshell
5
What does Pauli’s Exclusion Principle say?
A set of quantum numbers is specific to a certain electron, so no two electrons can have the same values for n, l, ml, and ms
What does Hund’s Rule say?
Orbitals may have identical energy levels when they are of the same principal shell. These orbitals are called degenerate, or "equal energy.”
So electrons fill orbitals one at a time (half fill all in that subshell before full filling).
What does the Heisenberg Uncertainty Principal say?
we cannot precisely measure the momentum and position of an electron at the same time
Where do nodes occur?
occurs at points where the wave function is zero and changes signs