Organic Chemistry Reactions
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
43 Terms
H-X
X adds Markovnikov
H-Br and ROOR
X adds anti-Markovnikov
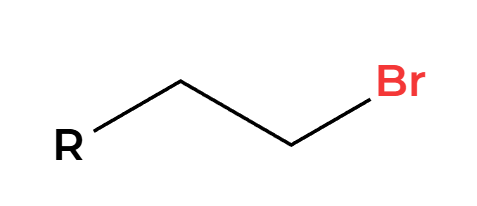
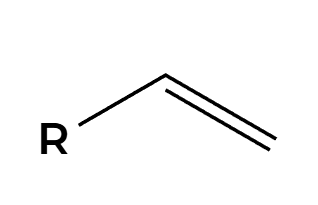
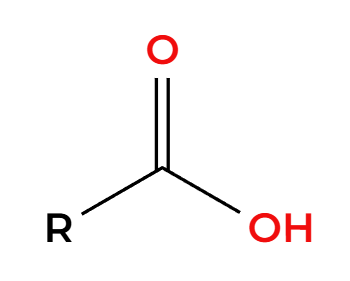
H+ and H2O
OH adds Markovnikov
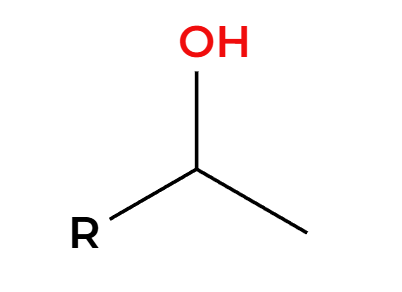
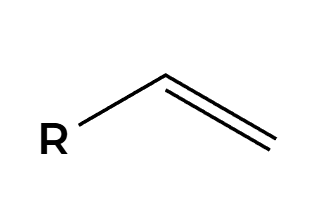
H3O+
OH adds Markovnikov
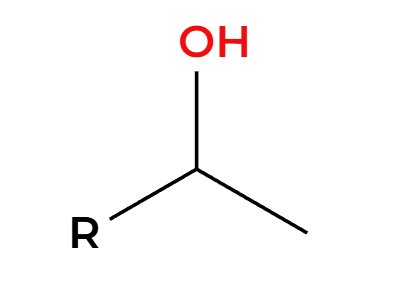
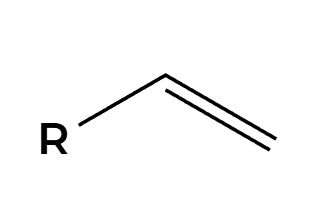
H2SO4 (dilute)
OH adds Markovnikov
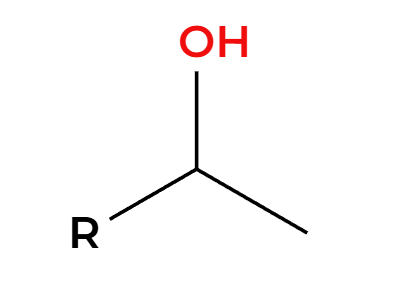
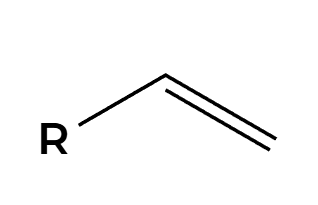
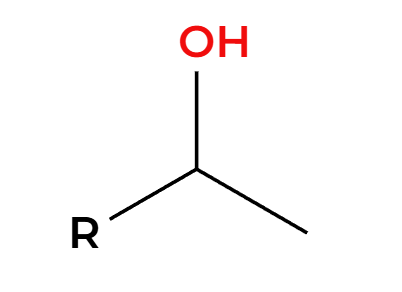
Hg(OAc)2, H2O
NaBH4
OH adds Markovnikov
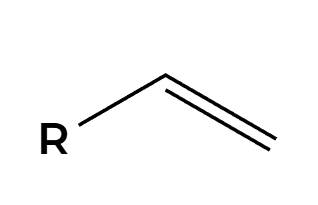
BH3
H2O2, NaOH
OH adds Anti-Markovnikov, groups add syn
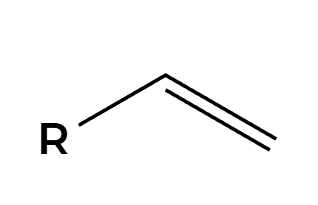
H2, Pt
H’s add syn
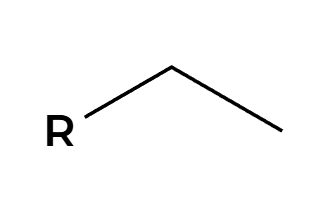
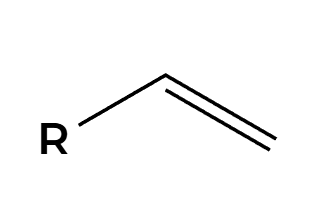
X2
X’s add anti
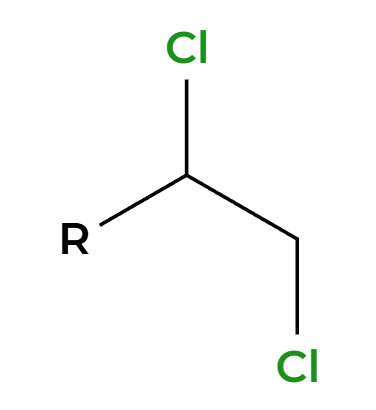
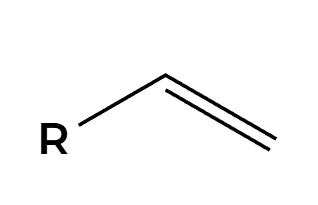
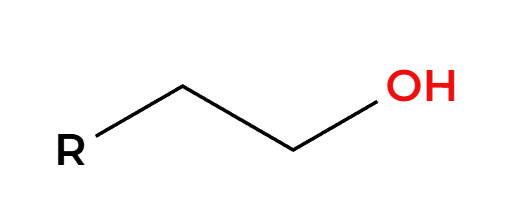
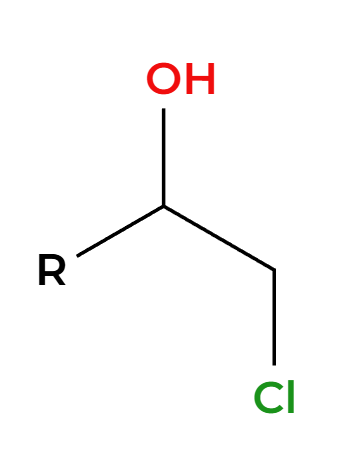
X2, H2O
OH adds Markovnikov, add anti
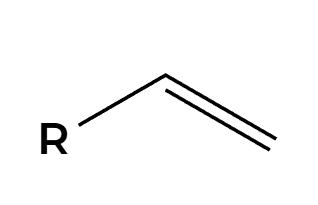
RCO3H
H3O+
OH’s add anti
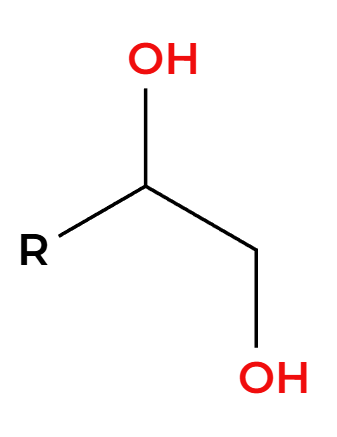
OsO4
NaHSO3, H2O
OH’s add syn
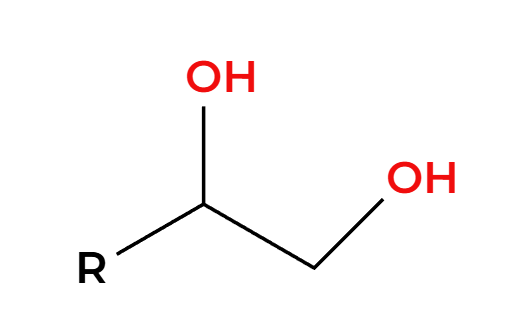
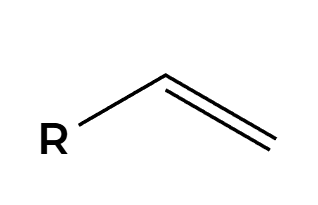
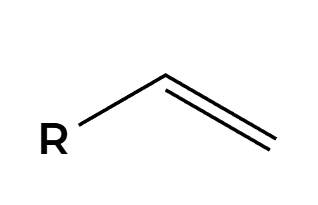
MCPBA
H3O+
OH’s add anti
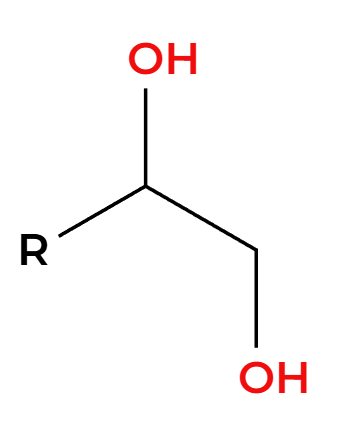
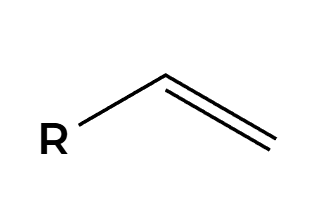
O3
DMS
cleavage
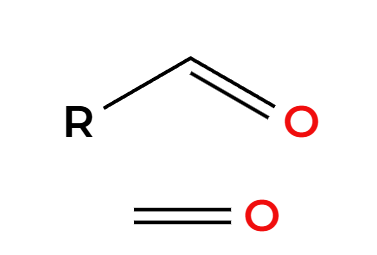
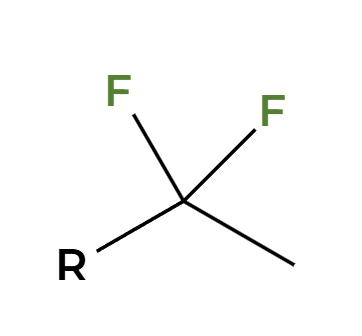
NaNH2
alkyne
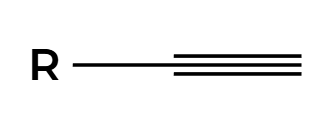
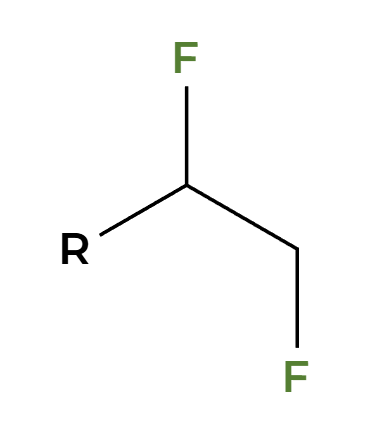
NaNH2
alkyne
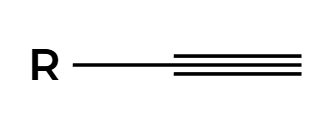
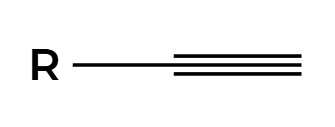
HX
X adds Markovnikov
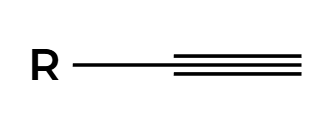
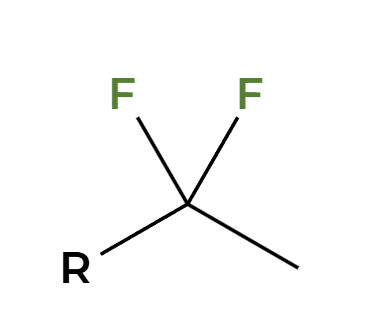
2HX
2X adds Markovnikov
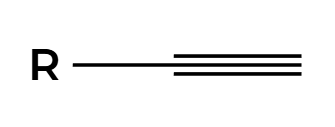
2X2
add 2 to each side of pi bonds
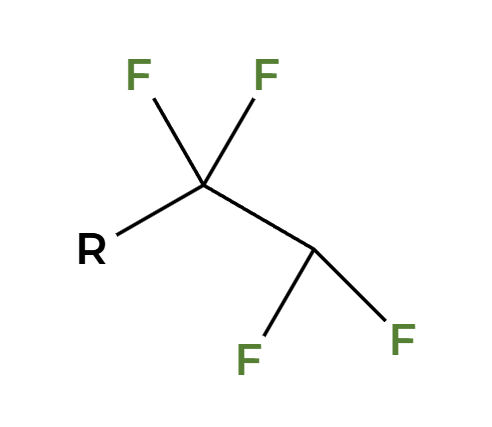
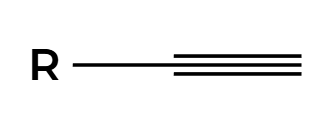
X2
Xs add anti
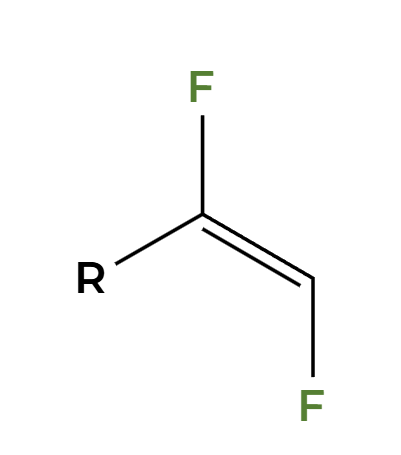
HgSO4, H2SO4, H2O
OH adds Markovnikov to make enol then tautomerization to ketone
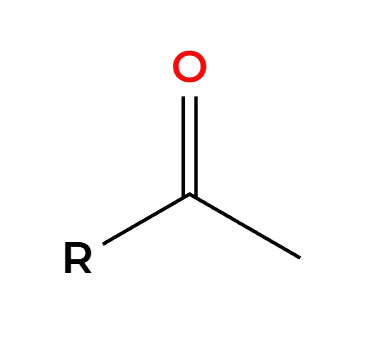
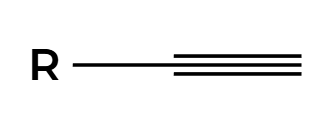
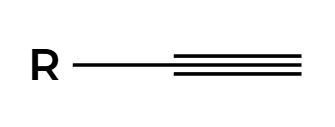
R2BH
H2O2, NaOH
OH adds anti Markovnikov to make enol then tautomerization to aldehyde
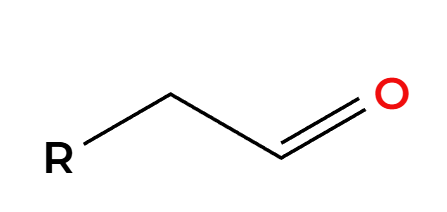
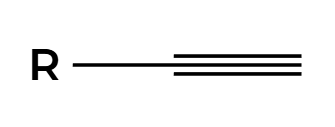
H2, Pt
cannot stop at alkene, goes all the way to alkane
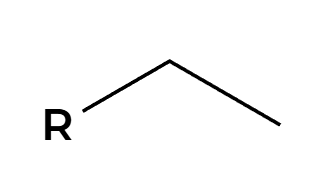
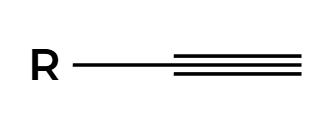
H2, Lindlar
H’s add syn
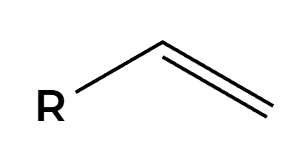
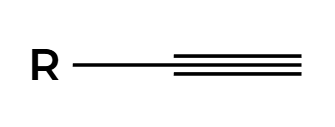
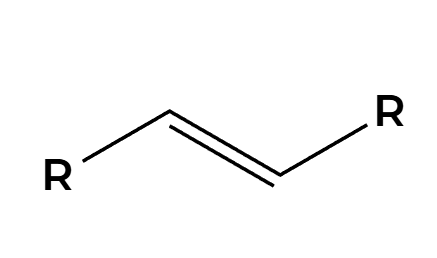
Na, NH3
H’s add anti
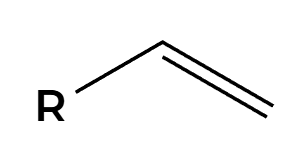
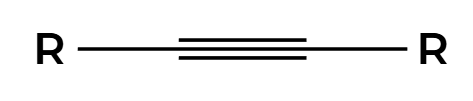
H2, Lindlar
only cis product is made
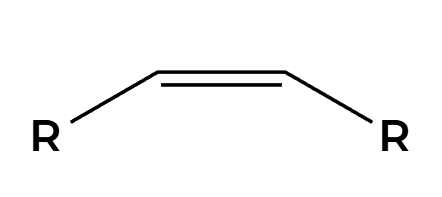
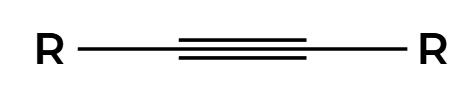
Na, NH3
only trans product is made
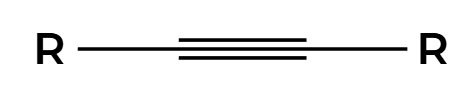
O3
H2O
ozonolysis
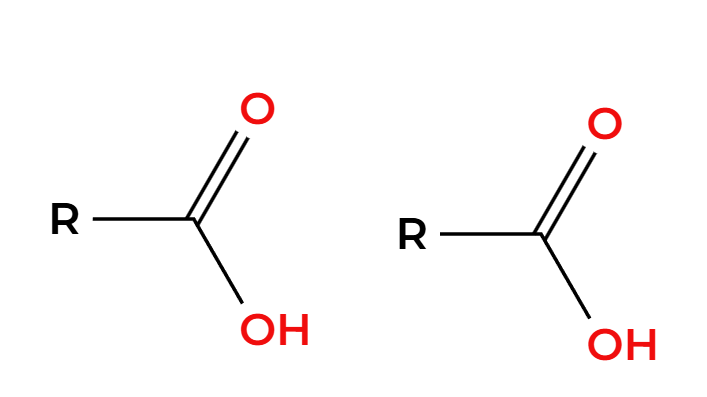
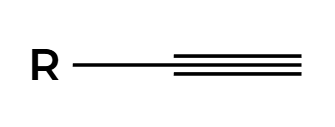
O3
H2O
ozonolysis
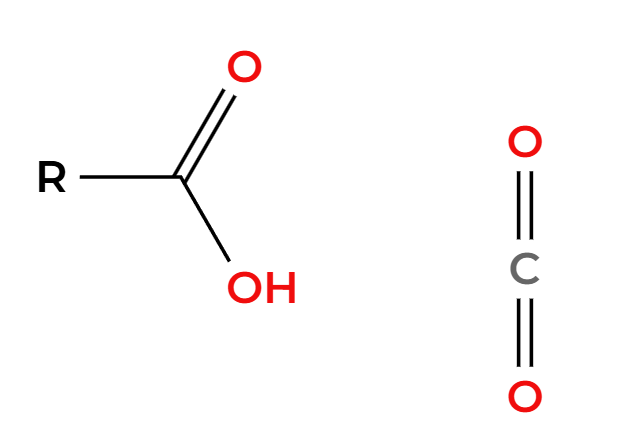
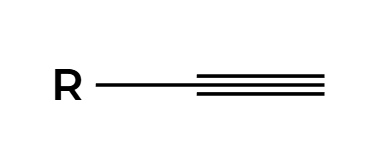
NaNH2
low substituted alpha carbon
coupling reaction
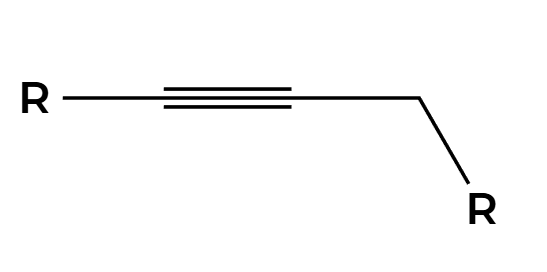
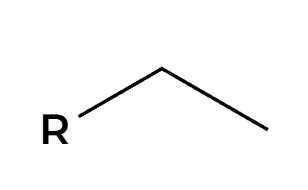
Br2 and light (hv)
Br adds most substituted
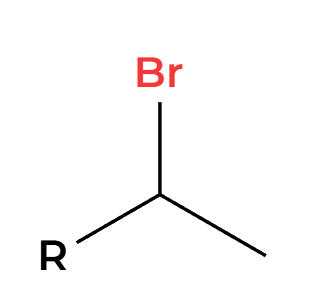
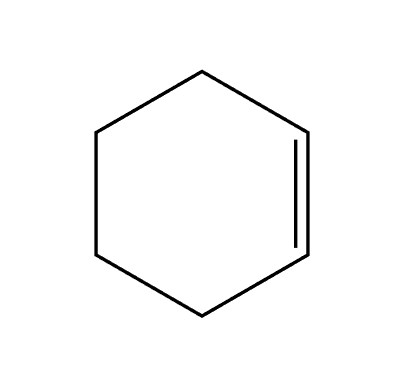
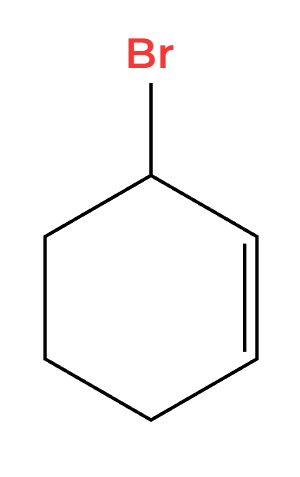
NBS and light (hv)
Br adds more substituted allylic
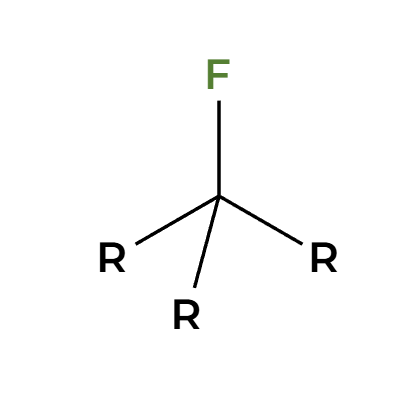
H2O
only works on tertiary alpha C
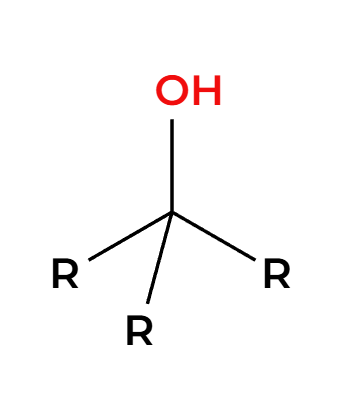
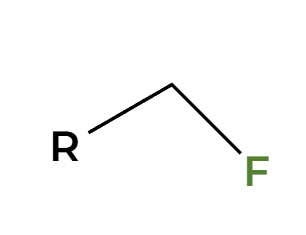
NaOH
only works on primary alpha C
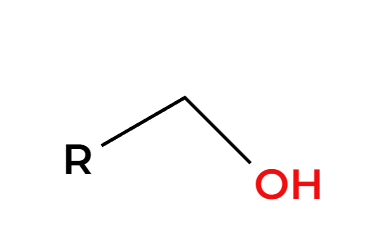
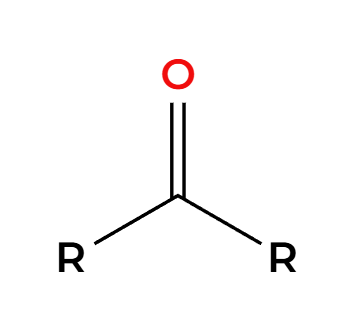
NaBH4 and ROH
mild reduction
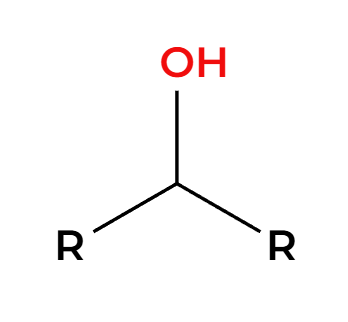
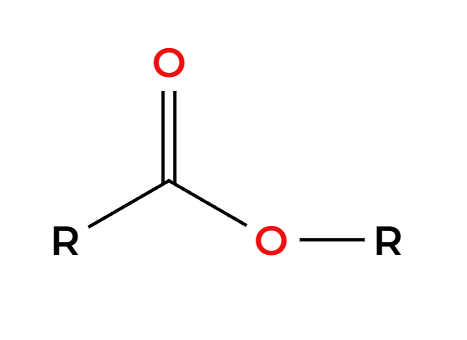
LAH
H2O
strong reduction
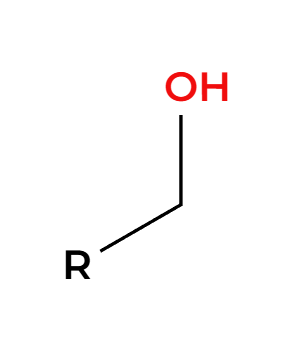
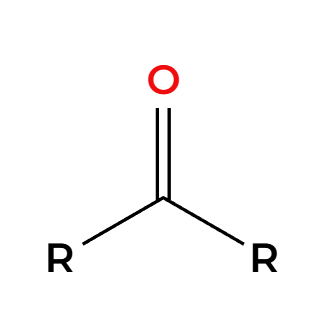
RMgBr
H2O
reduction and coupling
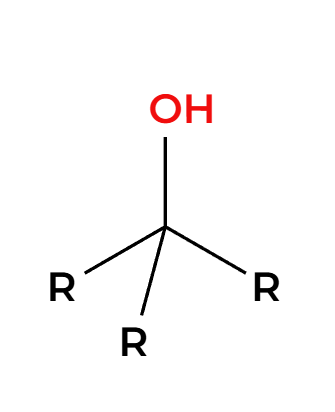
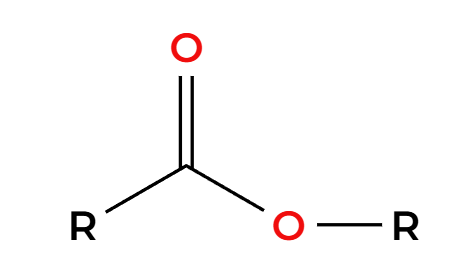
RMgBr
H2O
reduction and coupling
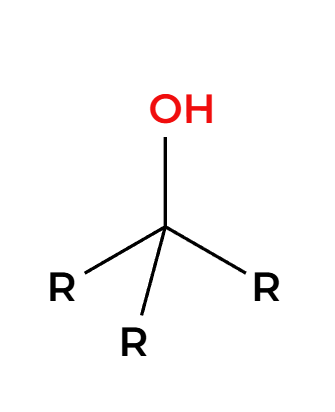
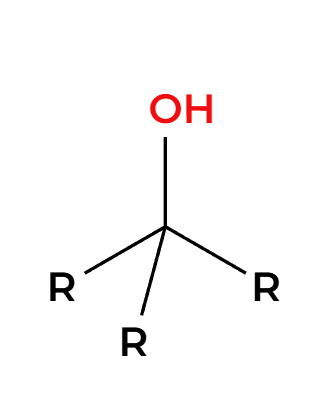
HX
only works on primary or tertiary alpha carbons
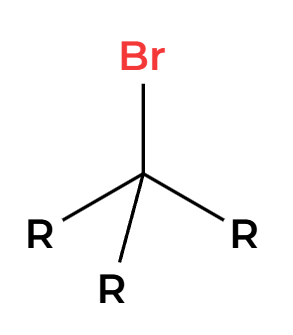
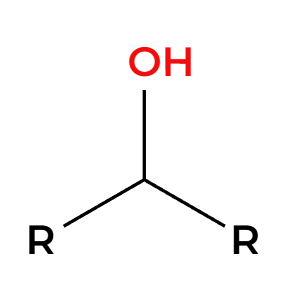
SOCl2
Cl adds 180 opposite
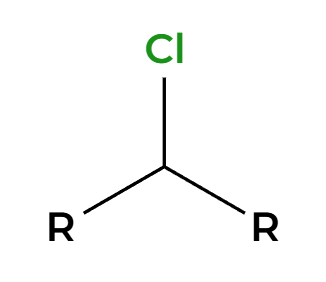
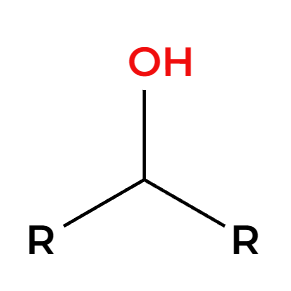
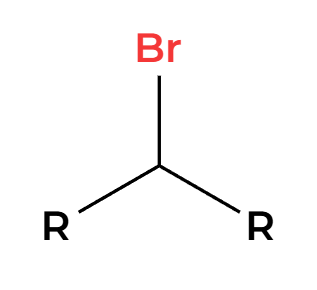
PBr3
Br adds 180 opposite
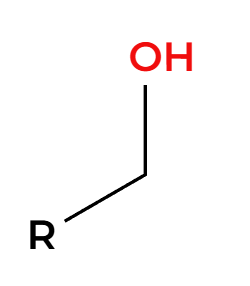
PCC
mild oxidation
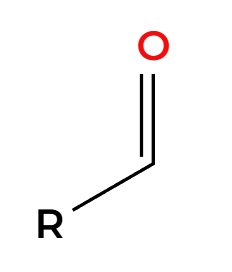
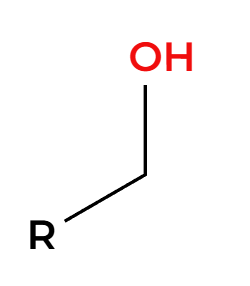
CrO3
strong oxidation