Chapter 13: Intercellular Membrane Traffic
Intercellular Membrane Traffic
- Every cell communicates with the world around it and quickly responds to changes within its environment.
- By what?
- Exocytosis: The secretory pathway delivers newly synthesized proteins, carbohydrates, and lipids either to the plasma membrane or extracellular space.
- Endocytosis: The cells remove plasma membrane components and deliver them to internal compartments called endosomes, from where they can be recycled to the same or different regions of the plasma membrane or be delivered to lysosomes for degradation.
The Molecular mechanisms of membrane transport and the maintenance of compartmental diversity
Coated vesicles
- Specialized coated regions of membrane bud off as coated vesicles.
- There are three well-characterized types of coated vesicles, distinguished by their major coat proteins:
- Clathrin-coated: Mediates transport from the Golgi apparatus and from the plasma membrane.
- COPI-coated: Mediate transport from the ER and from the Golgi cisternae.
- COPII-coated: Mediate transport from the ER and from the Golgi cisternae.
Phosphoinositide mark organelles and membrane domains
- Comprise less than 10% of the total phospholipids in a membrane.
- They have important regulatory functions.
- Different organelles in the endocytic and secretory pathways have distinct sets of PI and PIP kinases and PIP phosphatases.
- The PIP-binding proteins then help regulate vesicle formation and other steps in the control of vesicle traffic.
- The forces generated by clathrin coat assembly alone are not sufficient.
- Membrane-bending proteins that contain crescent-shaped domains, called BAR domains.
- Bind to and impose their shape on the underlying membrane via electrostatic interactions with the lipid head groups.
- Other BAR-domain proteins help in important in shaping the neck of a budding vesicle.
Cytoplasmic proteins regulate the pinching-off and uncoating of coated vesicles
- Soluble cytoplasmic proteins, including dynamin, assemble at the neck of each bud.
- Dynamin contains:
- PI(4,5) P2-binding domain, which tethers the protein to the membrane.
- GTPase domain, which regulates the rate at which vesicles pinch off from the membrane.
- Pinching-off process brings the two noncytosolic leaflets of the membrane into close proximity and fuses them.
- Auxilin, another vesicle protein, is thought to activate the ATPase.
- The release of the coat, however, must not happen prematurely, so additional control mechanisms must somehow prevent the clathrin from being removed before it has formed a complete vesicle.
Monomeric GTPases Control Coat Assembly
- Local production of PIPs plays a major part in regulating the assembly of clathrin coats on the plasma membrane and Golgi apparatus.
- Coat-recruitment GTPases
- It controls the assembly of clathrin coats on endosomes and the COPI and COPII coats on Golgi and ER membranes.
- Members of the family of monomeric GTPases.
- Include
- ARF proteins which are responsible for the assembly of both COPI and clathrin coats assembly at the Golgi membrane.
- Sar1 protein, which is responsible for the assembly of COPII coats at the ER membrane.
Rab Proteins guide transport vesicles to their target membrane
- Rab proteins play a central part in the specificity of vesicle transport.
- Each Rab protein is associated with one or more membrane-enclosed organelles of the secretory or endocytic pathways.
- Rab proteins can function on transport vesicles, on target membranes, or both.
- Rab proteins cycle between a membrane and the cytosol and regulate the reversible assembly of protein complexes on the membrane.
- Rab proteins bind to the other proteins called Rab effectors.
- Rab proteins differ greatly in structure and function.
- Rab cascades can change the identity of an organelle.
- The SNARE proteins (also called SNAREs, for short) catalyze the membrane fusion reactions in vesicle transport.
- 35 different SNAREs in an animal cell.
- Transmembranes exist as complementary sets:
- v-SNAREs are usually found on vesicle membranes.
- d t-SNAREs are usually found on target membranes.
Transport from the ER through the Golgi apparatus
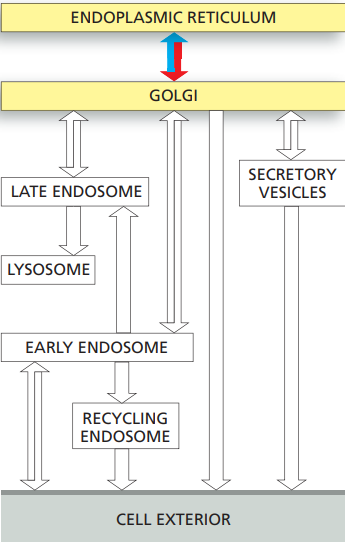
- Correctly folded and assembled proteins in the ER are packaged into COPII-coated transport vesicles that pinch off from the ER membrane.
- The vesicles shed their coat and fuse to form vesicular tubular clusters.
- In animal cells, the clusters then move on microtubule tracks to the Golgi apparatus, where they fuse to form the cis Golgi network.
- Any resident ER proteins that escape from the ER are returned there from the vesicular tubular clusters and Golgi apparatus by retrograde transport in COPI-coated vesicles.
- The Golgi apparatus, unlike the ER, contains many sugar nucleotides, which glycosyl transferase enzymes use to glycosylate lipid and protein molecules as they pass through the Golgi apparatus.
- The mannoses on the N-linked oligosaccharides that are added to proteins in the ER are often initially removed, and further sugars are added.
- The Golgi apparatus is the site where O-linked glycosylation occurs and where glycosaminoglycan chains are added to core proteins to form proteoglycans.
- Sulfation of the sugars in proteoglycans and of selected tyrosines on proteins also occurs in a late Golgi compartment.
The Golgi apparatus
- It modifies the many proteins and lipids that it receives from the ER and then distributes them to the plasma membrane, lysosomes, and secretory vesicles.
- The Golgi apparatus is a polarized organelle, consisting of one or more stacks of disc-shaped cisternae.
- Each stack is organized as a series of at least three functionally distinct compartments, termed cis, medial, and trans cisternae.
- The cis and trans cisternae are each connected to special sorting stations, called the cis Golgi network and the trans-Golgi network, respectively.
- Proteins and lipids move through the Golgi stack in the cis-to-trans direction.
- Continual retrograde vesicle transport from upstream to downstream cisternae.
- The finished new proteins end up in the trans-Golgi network, which packages them in transport vesicles and dispatches them to their specific destinations in the cell.
Transport from the trans-Golgi network to Lysosmes
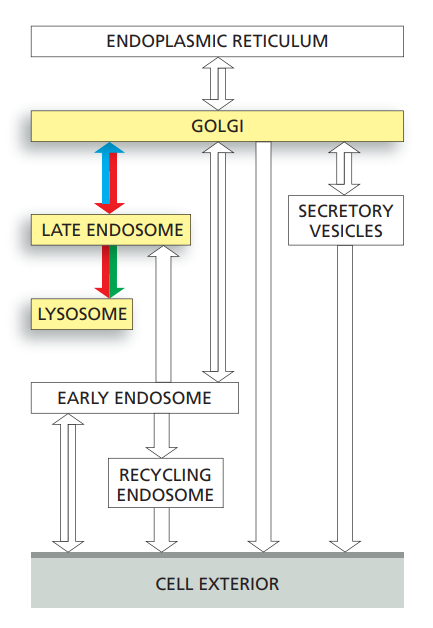
Lysosomes
- Lysosomes are membrane-enclosed organelles.
- It is filled with soluble hydrolytic enzymes that digest macromolecules.
- It contains about 40 types of hydrolytic enzymes, including proteases, nucleases, glycosidases, lipases, phospholipases, phosphatases, and sulfatases.
- Maintains interior pH of about 4.5–5.0.
- Lysosomes are heterogeneous.
- Plant and fungal vacuoles are remarkably versatile vacuoles.
- An ATP-driven H+ pump in the lysosomal membrane maintains this low pH.
- Newly synthesized lysosomal proteins are transported from the lumen of the ER, through the Golgi apparatus; they are then carried from the trans-Golgi network to endosomes using clathrin-coated transport vesicles, before moving on to lysosome.
How?
- The lysosomal hydrolases contain N-linked oligosaccharides that are covalently modified in a unique way in the cis Golgi so that their mannoses are phosphorylated.
- These mannose 6-phosphate (M6P) groups:
- They are recognized by an M6P receptor protein in the trans-Golgi network that segregates the hydrolases.
- It also helps package them into building transport vesicles that deliver their contents to endosomes.
- It shuttles back and forth between the trans-Golgi network and the endosomes.
- The low ph in endosomes and the removal of the phosphate from the M6P group cause the lysosomal hydrolases to dissociate from these receptors, making the transport of the hydrolases unidirectional.
- A separate transport system uses clathrin-coated vesicles to deliver resident lysosomal membrane proteins from the trans-Golgi-network to endosomes.
Transport into the cell from the plasma membrane: endocytosis
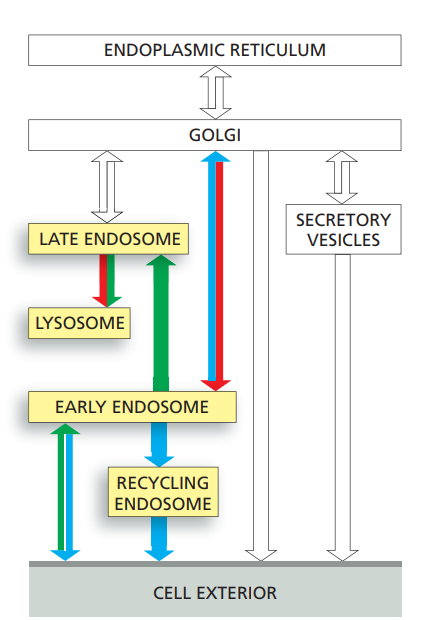
- Cells ingest fluid, molecules, and particles by endocytosis, in which localized regions of the plasma membrane invaginate and pinch off to form endocytic vesicles.
- Endocytosis internalizes a large fraction of the plasma membrane every hour.
- The cells remain the same size because most of the plasma membrane components (proteins and lipids) that are endocytosed are continually returned to the cell surface by exocytosis.
- This large-scale endocytic–exocytic cycle is mediated largely by clathrin-coated pits and vesicles but clathrin-independent endocytic pathways also contribute.
- While many of the endocytosed molecules are quickly recycled to the plasma membrane, others eventually end up in lysosomes, where they are degraded.
- The ligands that are endocytosed with their receptors dissociate from their receptors in the acidic environment of the endosome and eventually end up in lysosomes, while most of the receptors are recycled via transport vesicles back to the cell surface for reuse.
- Cell-surface signaling receptors become tagged with ubiquitin when activated by binding their extracellular ligands.
- Ubiquitylation guides activated receptors into clathrin-coated pits, they and their ligands are efficiently internalized and delivered to early endosomes.
Early endosomes → Late endosomes
- Early endosomes, rapidly mature into late endosomes.
- During maturation, patches of the endosomal membrane containing ubiquitylated receptors invaginate and pinch off to form intraluminal vesicles.
- This process is mediated by ESCRT complexes and sequesters the receptors away from the cytosol, which terminates their signaling activity.
- Late endosomes migrate along microtubules toward the interior of the cell where they fuse with lysosomes to form endolysosomes, where degradation occurs.
Transcytosis
- Both receptor and ligand are transferred to a different plasma membrane domain, causing the ligand to be released at a different surface from where it originated, a process called transcytosis.
Transport from the trans-Golgi network to the cell exterior: exocytosis
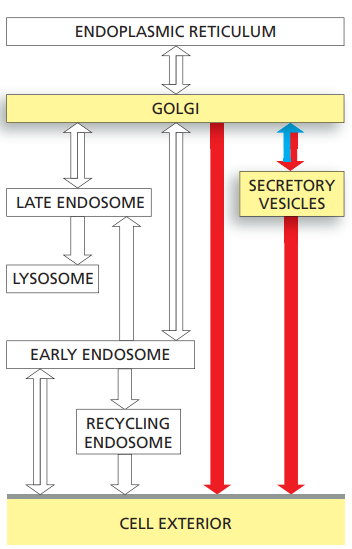
- Cells can secrete molecules by exocytosis in either a constitutive or a regulated fashion.
- Whereas the regulated pathways operate only in specialized secretory cells:
- In a constitutive secretory pathway that operates in all eukaryotic cells.
- Characterized by continual vesicle transport from the TGN to the plasma membrane.
- In a regulated pathway:
- The molecules are stored either in secretory vesicles or in synaptic vesicles, which do not fuse with the plasma membrane to release their contents until they receive an appropriate signal.
- Secretory vesicles containing proteins for secretion bud from the TGN.
- The secretory proteins become concentrated during the formation and maturation of the secretory vesicles.
- Synaptic vesicles, which are confined to nerve cells and some endocrine cells
- It forms from both endocytic vesicles and endosomes.
- They mediate the regulated secretion of small-molecule neurotransmitters at the axon terminals of nerve cells.
- Proteins are delivered from the TGN to the plasma membrane by the constitutive pathway unless they are diverted into other pathways or retained in the Golgi apparatus.



