6: Cell Function
Movement of substances through cell membranes
Cell Membrane Transport
Cells need to move substances to where they are needed.
Cytoskeleton for organelle movement, passive and active processes for moving substances across the membrane.
Passive Processes — no energy expenditure; particles move using their own energy.
Active Processes — requires energy; cells expends more metabolic energy to “carry“ particles across the membrane.
Passive Transport Processes
PROCESS | DESCRIPTION | EXAMPLES |
Simple diffusion | Movement of particles through the phospholipid bilayer or through channels from an area of high concentration to an area of low concentration—that is, down the concentration gradient | Movement of carbon dioxide out of all cells |
Osmosis | Passive transport of water through a selectively permeable membrane in the presence of at least one impermeant solute | Osmosis of water molecules into and out of cells to correct imbalances in water concentration |
Channel-mediated passive transport (facilitated diffusion) | Diffusion of particles through a membrane by means of channel structures in the membrane (particles move down their concentration gradient) | Diffusion of sodium ions into nerve cells during a nerve impulse |
Carrier-mediated passive transport (facilitated diffusion) | Diffusion of particles through a membrane by means of carrier structures in the membrane (particles move down their concentration gradient) | Diffusion of glucose molecules into most cells |
Diffusion
Diffusion — a natural phenomenon caused by the tendency of small particles to spread out evenly within any given space.
As molecules collide to one another, they tend to spread out, or diffuse.
Molecules move from an area of higher concentration to an area of lower concentration — thus spreading down the concentration gradient.
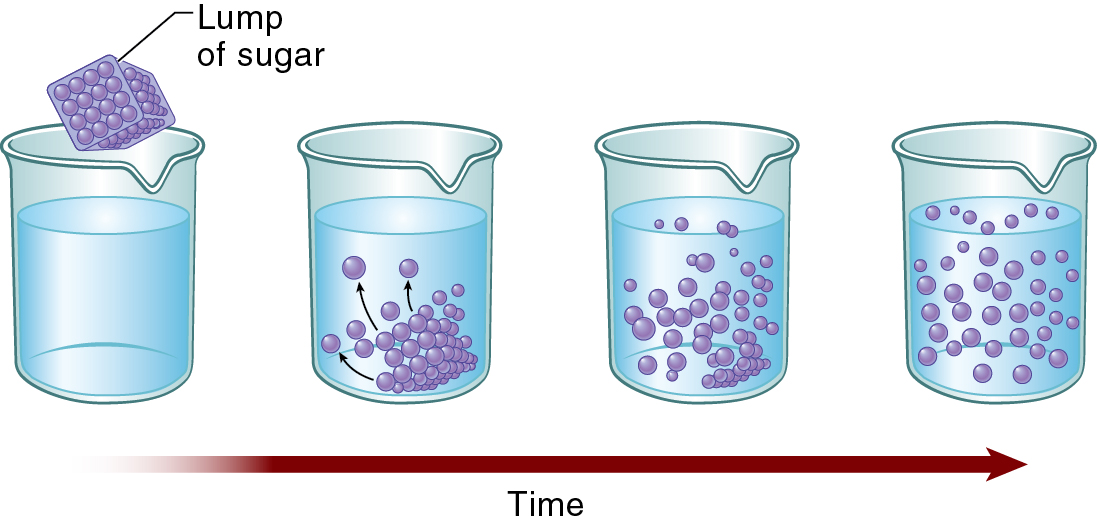
Concentration Gradient — a measurable difference in concentration from one area to another.
The greater the difference in concentration from one area to another, the greater is the movement of the molecules.
Equilibrium occurs when both solutions have equal concentrations.
Dynamic equilibrium — a balanced state in which the number of molecules of a substance bouncing to one side of the membrane exactly equals the number of molecules of that substance that are bouncing to the other side.
Once equilibration has occurred, overall diffusion may have stopped, but balanced diffusion of small numbers of molecules may continue.
Simple Diffusion
Simple Diffusion — a process when molecules pass directly through the phospholipid membrane.
When molecules are allowed to cross a membrane, they are said to permeate the membrane.
A membrane is permeable to a molecule only if it can pass through that membrane.
A molecule is permeant if it is able to diffuse across a membrane, and it is impermeant if it is unable to diffuse across the membrane.
Dialysis — a form of diffusion in which the selectively permeable nature of a membrane causes the separation of smaller solute particles from larger solute particles.
Solutes — the particles dissolved in a solvent.
Solution — a mixture of solute and solvent.
A bag made of dialysis membrane—material with microscopic pores—is filled with a solution containing glucose, water, and albumin (protein) molecules and immersed in a container of pure water. Smaller molecules are able to pass through pores while the larger solutes remain inside the bag.

Haemodialysis — blood pumped from a patient is exposed to a dialysis membrane that separates the blood from a clean, osmotically balanced dialysis fluid.
The process of dialysis is used to “clean up” the patient’s blood because the kidneys have failed to perform this task.
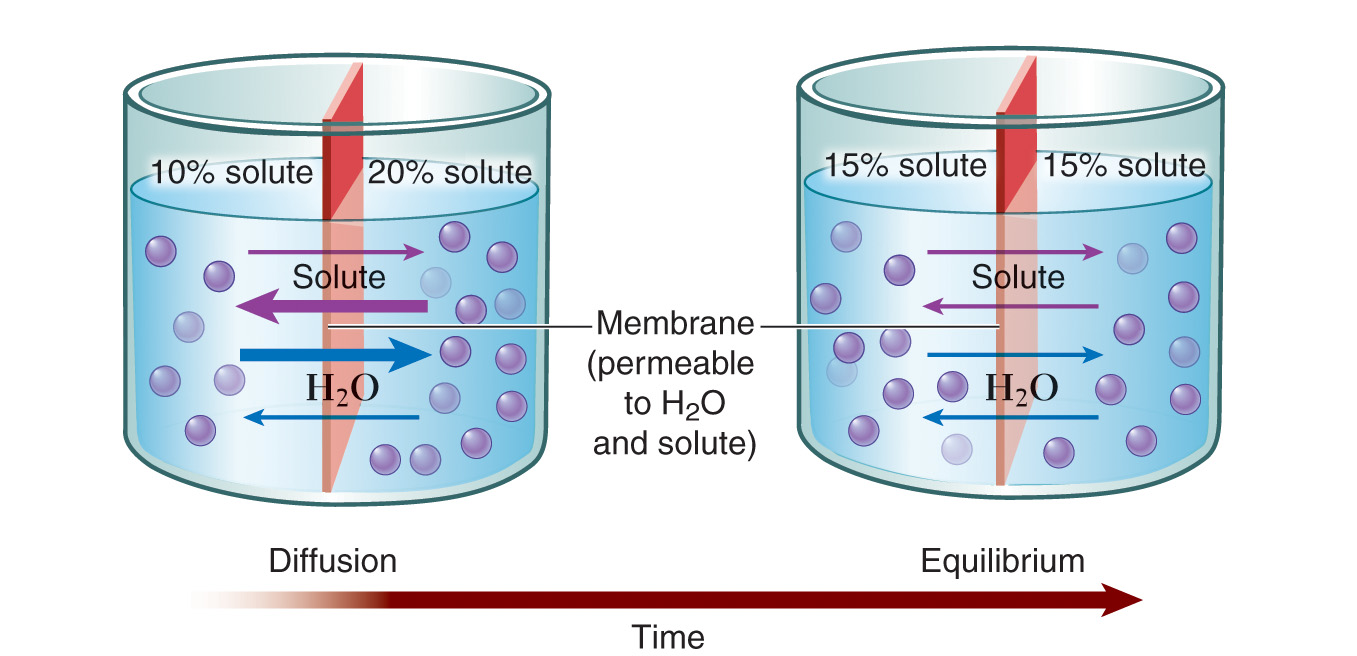
Osmosis
Osmosis — the passive movement of water through a selectively permeable membrane.
Water is permeant and therefore can equilibrate its concentration on both sides of the membrane.
Aquaporins — small water channels in cell membranes, making it permeable to water.
Also called aquapores.
The water molecules move or osmose through the membrane from the area of high water concentration to the area of low water concentration.
Only the water moves. One solution loses volume, one solution gains volume.
Osmotic pressure — the water pressure that develops in a solution as a result of osmosis.
Potential osmotic pressure — the maximum osmotic pressure that could develop in a solution when it is separated from pure water by a selectively permeable membrane.
Actual osmotic pressure — the pressure that already has developed in a solution by means of osmosis, it is easy to measure because it is already there.
When cells are in an isotonic solution, there will be no net movement of water. It does not cause cell swelling or shrinkage.
When cells are in a hypertonic solution, it causes water to move out of cells—leading to cell shrinkage.
If cells shrivel too much, they will be permanently damaged.
When human cells are placed in a very dilute solution, the cell may swell.

Facilitated diffusion
Facilitated Diffusion — this transport requires membrane transporters to help substances cross the membrane.
Ions (like Na+) and small molecules (like glucose) can pass through cell membranes with the help of these transporters.
Channel-mediated passive transport — a passive diffusion where ions move down their concentration gradients as they pass through channels.
Membrane channels — These are pores through which water molecules, specific ions, or other small, water-soluble molecules can pass.
These channels can exhibit specificity because their molecular structure prevents molecules of the wrong shape and pattern of charges to pass through the channel.
They are also called gated channels—as then opens and closes.
Some of these channels are triggered by electrical charges, light, mechanical or chemical stimuli.
Because a living cell membrane can limit the diffusion of some molecules by opening or closing channels in different situations, the membrane is selectively permeable.
Carrier-mediated passive transport — a process where membrane carriers facilitate diffusion. It involves membrane carriers that bind and transport molecules.
Membrane Carriers — Proteins that facilitate the diffusion of molecules down their concentration gradient.
Carrier protein attracts a solute molecule, changes shape, and releases the solute to the other side of the membrane.
Carrier-mediated transport can move molecules in either direction, depending on the concentration gradient.
It involves binding and shape changing.
Filtration
Filtration — this form of transport involves the passing of water and permeable solutes though a membrane by the force of hydrostatic pressure.
It is the movement of molecules though a membrane from an area of high hydrostatic pressure to an area of low hydrostatic pressure.
Hydrostatic pressure — the force of a fluid pushing against the surface.
Filtration is driven by hydrostatic pressure gradient.
Example:
Capillary filtration — this allows the blood vessels to supply tissues with water and other essential substances quickly and easily without losing its cells and blood proteins.
Role of passive transfer processes
Maintaining equilibrium: Passive transport helps to maintain the balance of substances inside and outside cells, ensuring optimal cellular function.
Nutrient uptake: Cells use passive transport to absorb essential nutrients from the extracellular environment.
Waste removal: Passive transport allows cells to eliminate waste products.
Signal transduction: Passive transport is involved in the movement of signaling molecules across the cell membrane, which is essential for cell communication and regulation.
Cellular homeostasis: Passive transport helps to maintain a stable internal environment within cells.
Active transport processes
Transport by pumps
Membrane pumps — these are membrane transporter that carry out transport process in which cellular energy is used to move molecules “uphill” through a cell membrane.
The substance moves from an area of low concentration to an area of higher concentration.
An actively transported substance moves against its concentration gradient.
Types of Transporters:
Uniporter — a type of transporter that transports only one type of molecule at a time.
Symporter — a type of transporter that move two or more types of molecule in the same direction through a membrane.
Also called as cotransport.
Antiporter — a type of transporter that move two different types of molecules in opposite directions at the same time.
Also called countertransport.
Primary Active Transport — this uses an ATP directly to pump substances against their concentration gradient.
Secondary Active Transport — this uses the energy stored in ion gradients created by primary active transport. This couples the movement of one substance down its concentration gradient to drive the movement of another substance against its gradient.
Active pumping — allows the cells to move certain ions or other water-soluble particles to specific areas and against their concentration gradient (requiring energy expenditure).
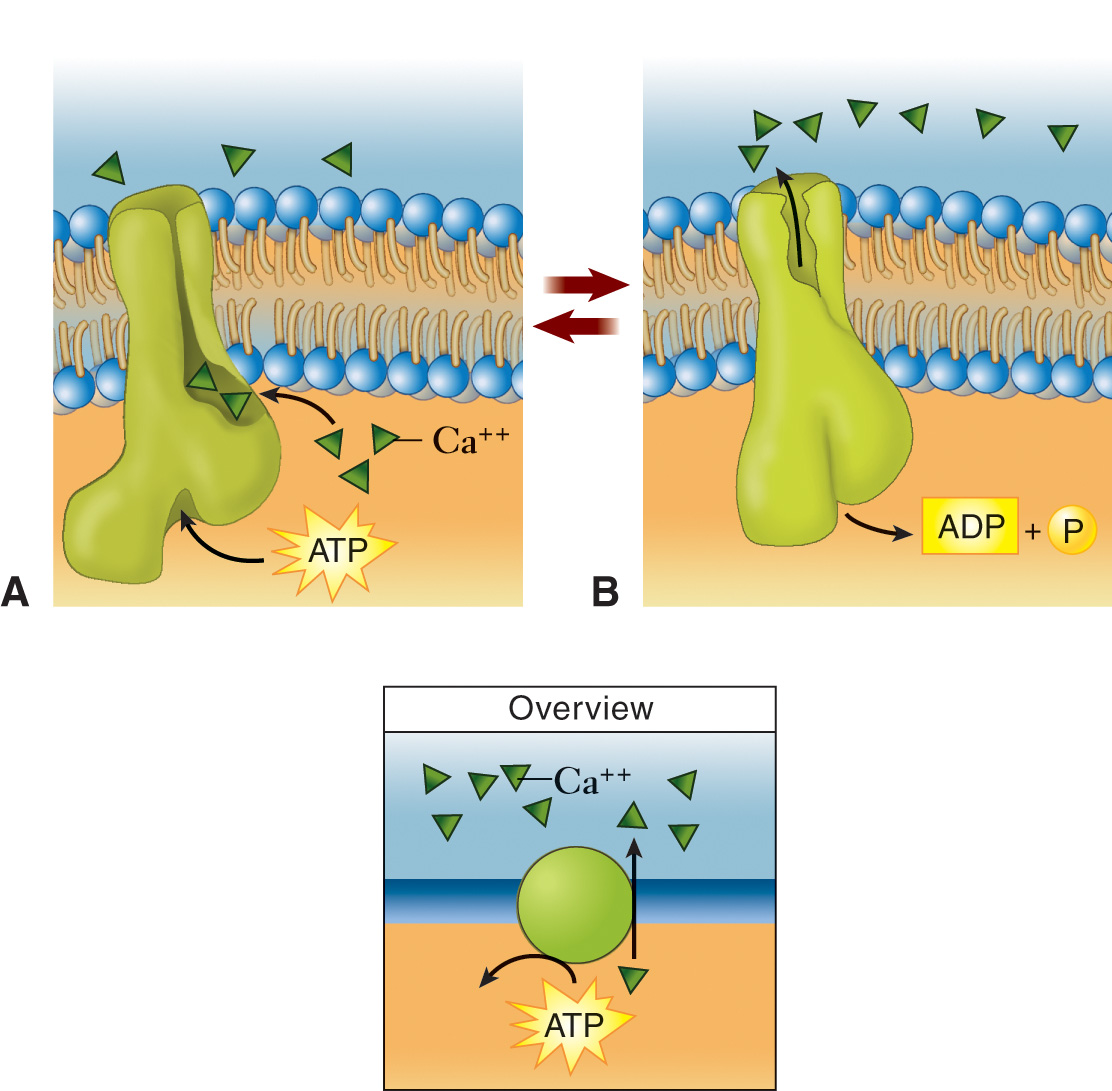
Calcium Pumps in Muscle Cells
During muscle relaxation, calcium pumps actively transport calcium ions out of the cell or into specialized compartments within the cell.
A low intracellular calcium concentration is essential for proper muscle function. By removing calcium, these pumps help to maintain this balance.
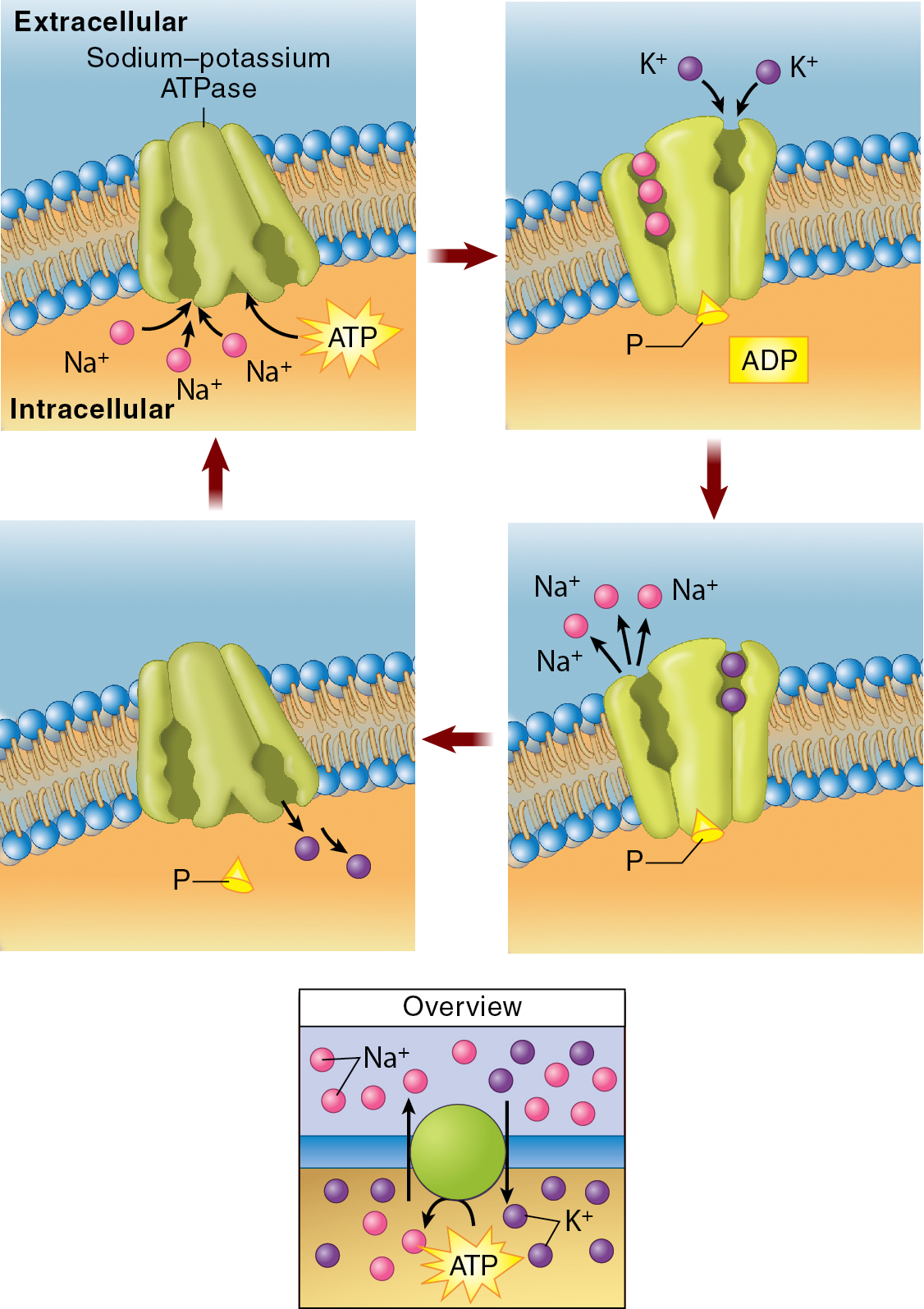
Sodium-Potassium Pump:
Found in the plasma membrane of all human cells.
Maintains specific concentration gradients of sodium and potassium ions across the cell membrane. It required energy from ATP.
It pumps sodium out and potassium in of the cell.
Low concentration of sodium, high concentration of potassium.
Mechanism:
Three sodium ions (Na+) bind to the pump's inner face, while an ATP molecule binds to the pump.
The ATP molecule breaks down, releasing energy that is used to change the pump's shape.
The pump releases the three sodium ions to the outside of the cell and attracts two potassium ions (K+) to its potassium-binding sites.
The pump returns to its original shape, releasing the two potassium ions and the remaining ATP molecule to the inside of the cell.
Importance:
Maintains cell volume
Nerve impulse transmission
Muscle contraction
Kidney Function
Transport by Vesicles
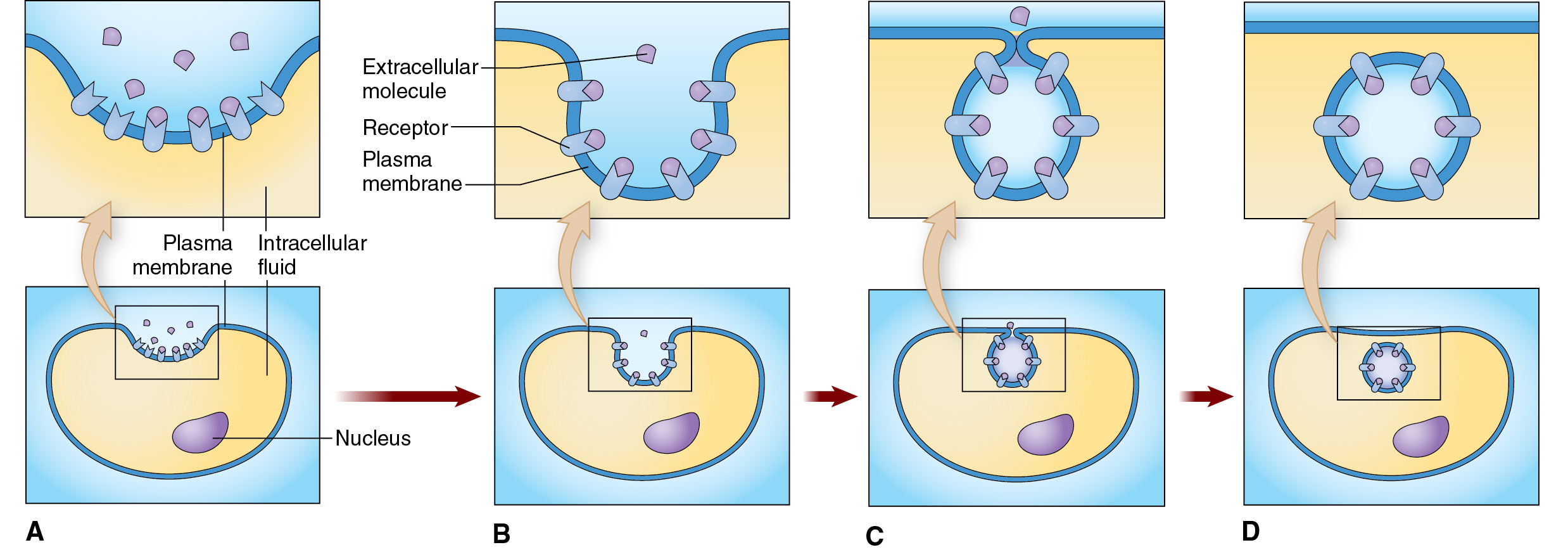
Endocytosis — a cellular process by which cells take in substances from the outside environment by engulfing them in a vesicle formed from the plasma membrane.
The plasma membrane forms a depression or pocket to enclose the substance.
The edges of the membrane fuse to create a vesicle.
The cytoskeleton plays a crucial role in pulling the membrane inward and forming the vesicle.
Types of Endocytosis:
Phagocytosis (Cell Eating) — This is a type of endocytosis where large particles, such as bacteria or cell debris, are engulfed by the cell.
Pinocytosis (Cell Drinking) — This is a type of endocytosis where small particles or fluid are taken in by the cell.
Receptor-mediated endocytosis — a specific type of endocytosis where cells take in substances from the outside environment by binding to specific receptors on their surface. It's like the cell has "docking stations" for specific molecules.
Feature | Phagocytosis | Pinocytosis |
Definition | Cell Eating | Cell Drinkin |
Material Engulfed | Large particles such as bacteria, dead cells, or debris | Small particles or liquids containing dissolved nutrients |
Vesicle Size | Large vesicles (phagosomes) | Small vesicles |
Energy Requirement | Requires more energy due to engulfing larger substances | Requires less energy as it deals with smaller substances |
Cell Type | Performed mainly by specialized cells like macrophages and neutrophils | Occurs in many types of cells |
Purpose | Used for immune defense, removing dead cells, and debris | Used to absorb nutrients and fluids from the environment |
Mechanism | The cell membrane extends pseudopods to surround and engulf the particle | The cell membrane forms invaginations to engulf fluid |
Example | Macrophages engulfing a pathogen | Absorption of nutrients by intestinal cells |
Exocytosis — the by which large molecules, notably proteins, can leave the cell even though they are too large to move out through the plasma membrane.
The molecules are first enclosed in vesicles by the Golgi apparatus.
The vesicles are transported to the plasma membrane by the cytoskeleton.
The vesicles fuse with the plasma membrane, releasing their contents outside the cell.
Exosomes — extracellular vesicles (EVs) that contain a variety of biomolecules.
It can transfer signaling molecules between cells, influencing their behavior and function.
It can participate in immune response by modulating the activity of immune cells.
Example of Exocytosis:
Hormone secretion
Neurotransmitter release
Enzyme secretion
Role of Active Transport Processes
Maintaining ion gradients: It is essential for maintaining the concentration gradients of ions across cell membranes, which is crucial for various cellular functions, such as nerve impulse transmission and muscle contraction.
Nutrient uptake: Cells use active transport to absorb nutrients from the extracellular environment, even when the concentration of the nutrient is lower outside the cell.
Waste removal: Active transport can be used to remove waste products from cells.
Cellular signaling: Active transport plays a role in cellular signaling by allowing cells to take in or release signaling molecules.
Organelle function: Active transport processes are involved in the functioning of various organelles, such as the endoplasmic reticulum and Golgi apparatus.
Cell Metabolism
Metabolism
Metabolism — the sum of all the chemical reactions that occur within a living organism.
Cell metabolism — the chemical reactions of the cell.
Metabolic pathway — A sequence of chemical reactions that converts a starting material into a product.
Catabolic pathway — A pathway that breaks down complex molecules into simpler ones, releasing energy.
Anabolic pathway — A pathway that builds up complex molecules from simpler ones, requiring energy.
Roles of Enzyme
Enzymes — These are specialized proteins that act as biological catalysts, accelerating chemical reactions within cells.
These reduce the activation energy required for a chemical reaction to occur.
They increase the rate of chemical reactions, allowing metabolic processes to occur.
They are highly specific, catalysing only particular reactions.
Their activity can be rehulated, allowing cells to control metabolic processes in response in changing conditions.
Catalyst — a chemical that reduces the amount of activation energy needed to start a chemical reaction.
Activation Energy — the minimum amount of energy that is required to activate molecules to a condition in which they can undergo chemical transformation.
Chemical Structure of Enzymes
Enzymes are usually tertiary or quartenry protein of complex shape. They consist of amino acids linked together by peptide bonds.
Cofactors —Non-protein components that are essential for the activity of some enzymes. They can be inorganic ions (e.g., metal ions) or organic molecules (e.g., vitamins).
Coenzymes — Organic cofactors, often derived from vitamins. Examples include NAD+, FAD, and Coenzyme A.
Active site — the region of the enzyme molecule that binds to the substrate — ensuring that the enzyme only interacts with its intended target.
Substrate — the molecule being acted upon by the enzyme.
Lock-and-Key Model:
The enzyme and substrate fit together like a lock and key, forming an enzyme-substrate complex.
The enzyme catalyzes the chemical reaction, converting the substrate into products.
The enzyme remains unchanged after the reaction, and the products are released.
Induced fit model — the enzyme may undergo conformational changes to accommodate the substrate or to facilitate the reaction.
Classification and naming of enzymes
Enzymes are classified and named based on the type of chemical reaction they catalyze. Two common systems are used:
Suffix -ase: The suffix "-ase" is added to the root name of the substrate or the type of reaction.
Sucrase catalyzes the hydrolysis of sucrose.
Reaction type: Enzymes can also be classified based on the type of reaction they catalyze.
Hydrolases catalyze hydrolysis reactions.
Intracellular vs. Extracellular Enzymes:
Intracellular enzymes: Most enzymes act within cells.
Extracellular enzymes: Digestive enzymes are an important example of extracellular enzymes, acting outside of cells in the digestive tract.
Groups of Enzymes:
Oxidation-reduction enzymes — These are known as oxidases, hydrogenases, and dehydrogenases.
Energy release for muscular contraction and all physiological work depends on these enzymes.
Hydrolyzing enzymes, or hydrolases — Digestive enzymes belong to this group.
The hydrolyzing enzymes are named after the substrate acted on, for example, lipase, sucrase, and maltase.
Phosphorylating enzymes — These add or remove phosphate groups and are known as phosphorylases or phosphatases.
Enzymes that add or remove carbon dioxide — These are known as carboxylases or decarboxylases.
Enzymes that rearrange atoms within a molecule — These are known as mutases or isomerases.
Hydrases — These add water to a molecule without splitting it, as do hydrolases.
General function of enzymes
Rate Control: Enzymes determine the rate at which metabolic reactions occur. By altering the activity of enzymes, cells can adjust the speed of metabolic processes to meet their needs.
Pathway Direction: Enzymes can also influence the direction of a metabolic pathway. For example, some enzymes catalyze reactions that convert a molecule A into molecule B, while others catalyze the reverse reaction, converting B back into A. By controlling the relative activities of these enzymes, cells can determine the net flow of molecules through the pathway.
Regulation: The activity of enzymes can be regulated through various mechanisms, including:
Allosteric regulation — Binding of a molecule at a site other than the active site can alter the enzyme's activity.
Covalent modification — Enzymes can be activated or inactivated by the addition or removal of chemical groups, such as phosphate groups.
Gene expression: Cells can control the production of enzymes by regulating gene expression.
Allosteric Regulation
Allosteric Regulation — a type of enzyme regulation in which a molecule binds to a site on the enzyme that is different from the active site.
Allosteric effectors: These are molecules that can alter the activity of an enzyme by binding to a site other than the active site.
Molecules: These can bind to allosteric sites and influence enzyme activity.
pH: Changes in pH can alter the chemical attractions within the enzyme molecule, affecting its shape and activity.
Temperature: Changes in temperature can also affect the enzyme's shape and activity.
Cofactors: The presence or absence of cofactors can influence enzyme activity.
Binding of an allosteric effector can cause a conformational change in the enzyme, affecting the shape of the active site.
This change in shape can either activate or inhibit the enzyme's catalytic activity.
Feedback inhibition — a type of enzyme regulation where the end product of a metabolic pathway inhibits an earlier enzyme in the pathway.
End-product inhibition — a chemical product at the end of a metabolic pathway binds to the allosteric site of one or more enzymes along the pathway that produced it and thereby inhibits the synthesis of more product.
Most enzymes catalyze a chemical reaction in both directions, the direction and rate of the reaction being governed by the law of mass action.
Enzymes are continually being destroyed and therefore have to be continually synthesized, even though they are not used up in the reactions they catalyze.
Many enzymes are synthesized as inactive proenzymes.
Kinases — substances that convert proenzymes to active enzymes. They activate enzymes through allosteric effect.
Kinase A — these type of kinase activate enzymes that regulate certain pathways after a hormonal signal is received by the cell.
Catabolism
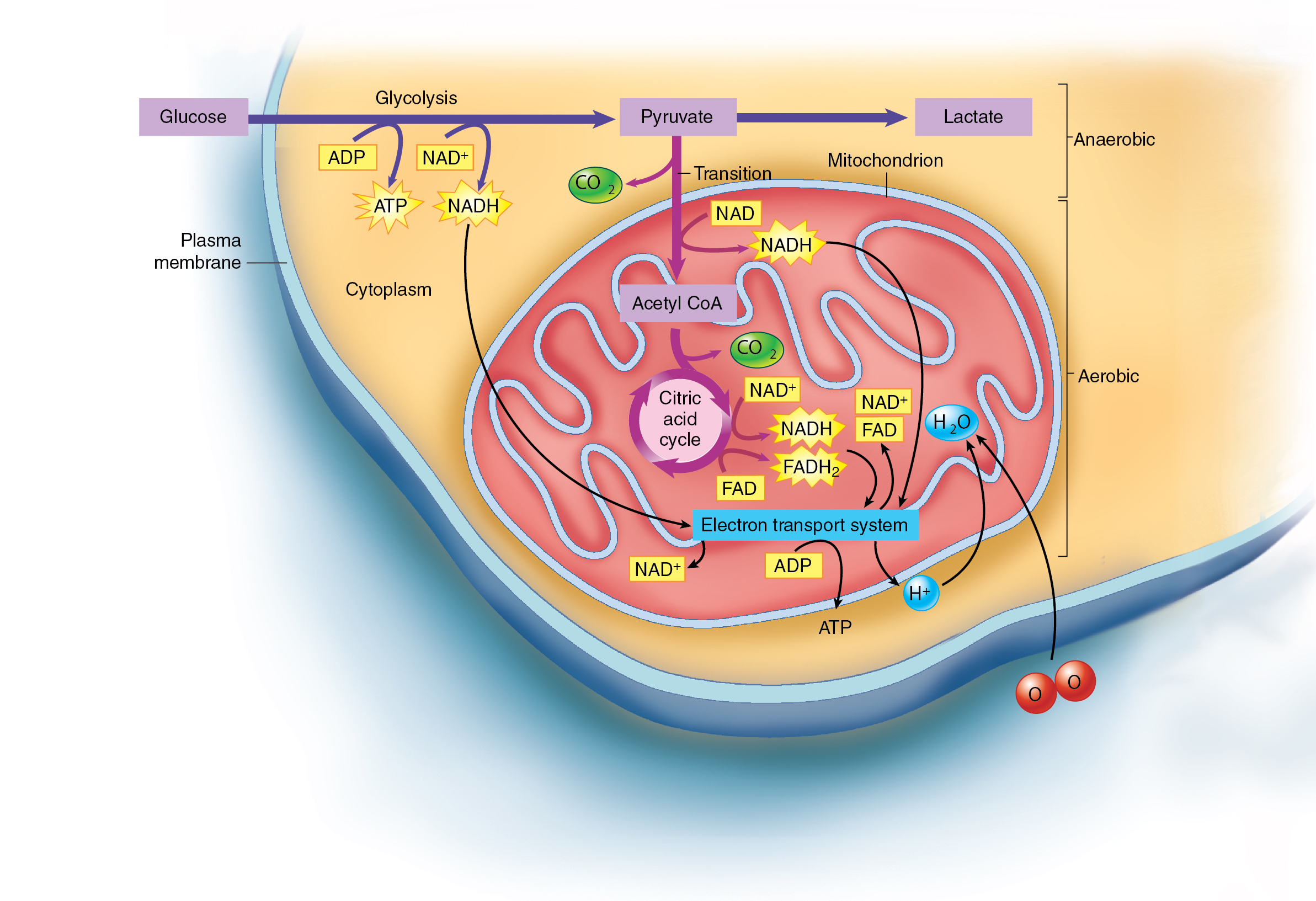
Cellular respiration — the process by which cells break down glucose into carbon dioxide and water.
Stages of Cellular Respiration:
Glycolysis: This occurs in the cytoplasm and breaks down glucose into pyruvate, producing a small amount of ATP.
Pyruvate oxidation: Pyruvate is converted into acetyl-CoA, which enters the mitochondria.
Citric acid cycle (Krebs cycle): Acetyl-CoA is further broken down, producing ATP, NADH, and FADH2.
Electron transport chain: NADH and FADH2 transfer electrons to the electron transport chain, which pumps protons across the inner mitochondrial membrane.
Oxidative phosphorylation: The energy from the proton gradient is used to produce ATP through oxidative phosphorylation.
Glycolysis
Glycolysis — a catabolic pathway that breaks down glucose into pyruvate, releasing a small amount of energy in the process. It also literally means “breaking glucose.“
It does not require oxygen.
It occurs in the cytosol of cells.
It produces ATP and NADH, which can be used for energy production.
Its final product is pyruvate—which can enter either aerobic or anaerobic pathway.
Aerobic Pathway:
Pyruvate enters the citric acid cycle, also known as the Krebs cycle or tricarboxylic acid (TCA) cycle.
Requires oxygen for the complete oxidation of pyruvate.
Produces a significant amount of ATP through oxidative phosphorylation.
Anaerobic Pathway:
Pyruvate is converted into lactate.
The accumulation of lactate due to insufficient oxygen availability is referred to as an oxygen debt or now more commonly as excess post-exercise oxygen consumption (EPOC).
Anaerobic glycolysis produces a smaller amount of ATP compared to the aerobic pathway.

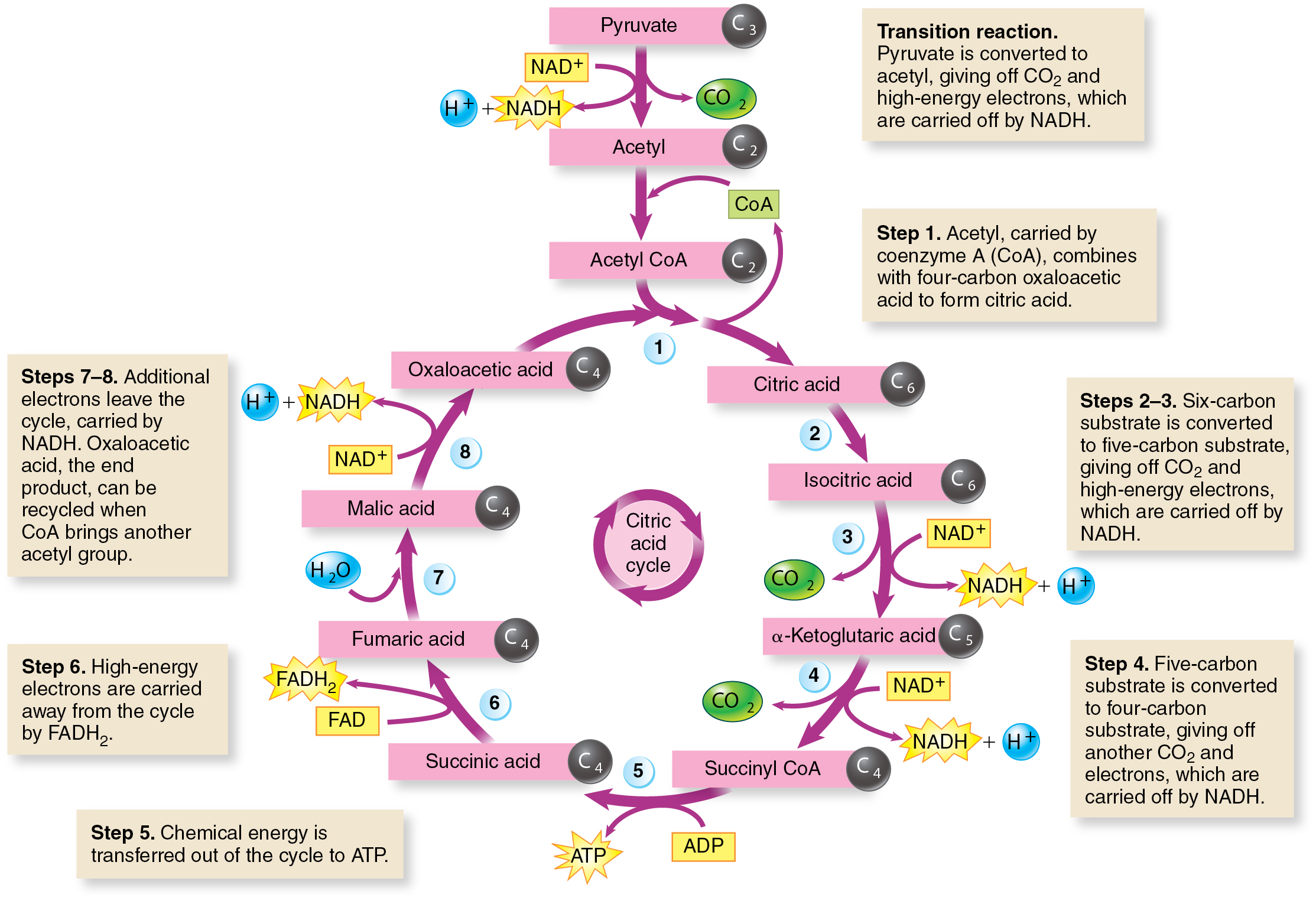
Citric Acid Cycle
Citric Acid Cycle — a central metabolic pathway that plays a crucial role in cellular respiration, that occurs in the mitochondria of cells and is responsible for further breaking down pyruvate, a product of glycolysis, to release energy.
It is also known as the Krebs cycle or tricarboxylic acid cycle.
Pyruvate is converted into acetyl-CoA before entering the cycle.
During the cycle, carbon dioxide is released as a waste product.
The cycle produces NADH and FADH2, which are electron carriers. These molecules carry high-energy electrons to the electron transport chain.
A small amount of ATP is produced directly in the citric acid cycle through substrate-level phosphorylation.
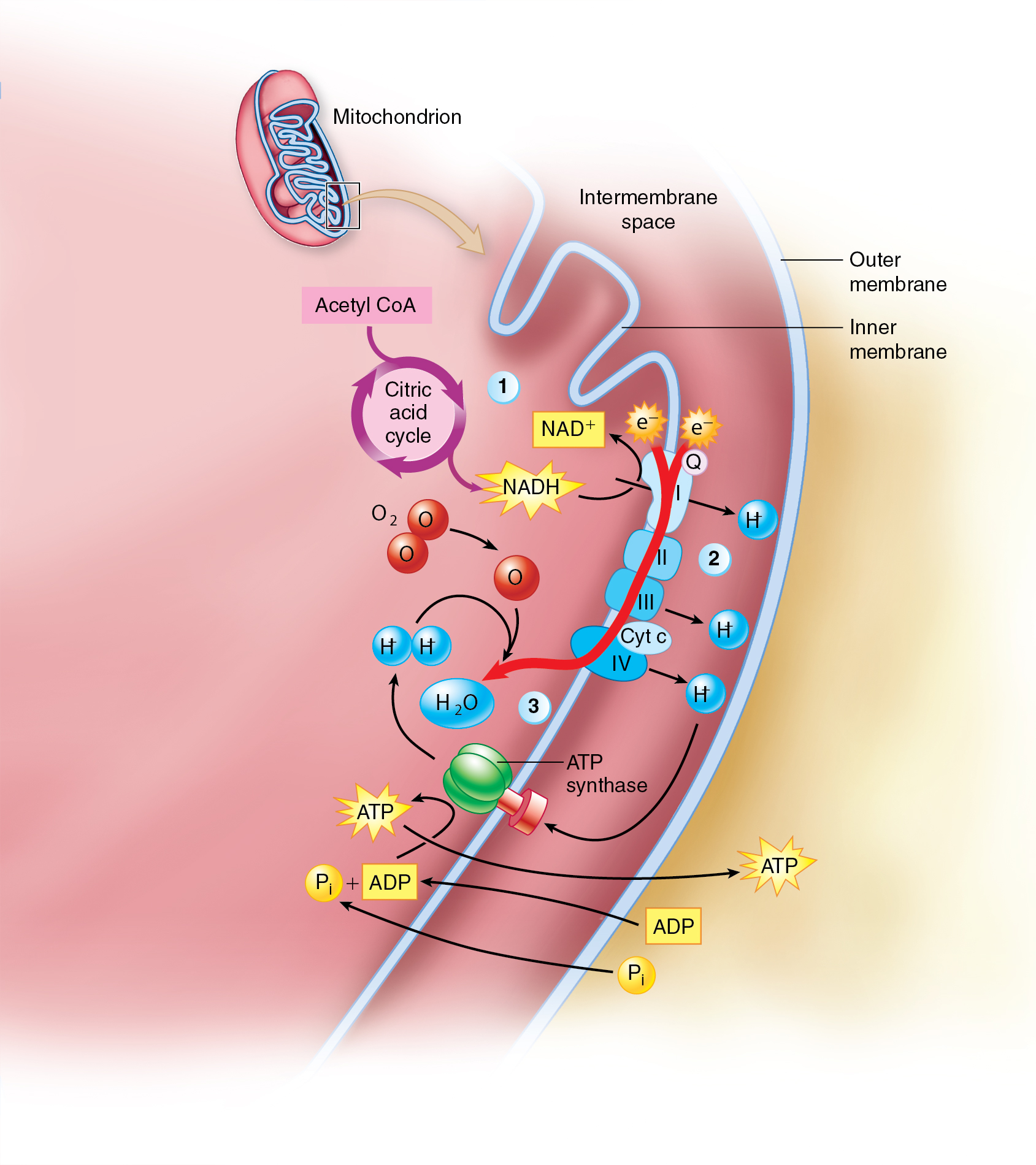
Electron transport system
ETS — the final stage of cellular respiration, occurring in the inner mitochondrial membrane.
It utilizes the energy stored in NADH and FADH2, produced in glycolysis and the citric acid cycle, to generate ATP.
High-energy electrons from NADH and FADH2 are transferred to a series of protein complexes embedded in the inner mitochondrial membrane.
As electrons move through the electron transport chain, energy is used to pump protons (H+) from the mitochondrial matrix into the intermembrane space, creating a proton gradient.
Protons flow back into the matrix through ATP synthase, a protein complex that harnesses the energy of this proton gradient to produce ATP.
Oxygen is required as the final electron acceptor, combining with electrons and protons to form water.
Anabolism
Anabolism — a type of metabolism that involves the building up of complex molecules from simpler ones.
It requires energy, often in the form of ATP (adenosine triphosphate).
It is essentially the process of synthesis, where smaller molecules are combined to form larger ones.
It requires energy to drive the chemical reactions involved in building complex molecules.
It include protein synthesis, muscle growth, and the formation of glycogen from glucose.
Cellular Physiology and the Whole Body
Cellular physiology — the study of how individual cells function. While it's important to understand the intricate details of cellular processes, it's also essential to consider how these processes work together to contribute to the overall function of the body.
Cells in the body work together in a coordinated manner to perform various functions.
Cells transcribe genes and synthesize proteins to carry out their specific roles.
Cells produce energy through processes like glycolysis, the citric acid cycle, and oxidative phosphorylation.
Cells transport materials into, out of, and within the cell using mechanisms like diffusion, active transport, and vesicular transport.
The cytoskeleton plays a crucial role in maintaining cell shape, facilitating cell movement, and organizing intracellular components.
Disorders of Cell Transport
Cystic Fibrosis (CF):
It is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CTFR) gene, which encodes a chloride ion (Cl-) channel protein.
In the most common form of CF, the CTFR protein is misfolded in the endoplasmic reticulum and is not transported to the plasma membrane.
This leads to abnormal Cl- transport, resulting in thick and sticky secretions in the lungs, pancreas, and other organs.
The thick mucus can block airways, leading to respiratory problems, and can also clog pancreatic ducts, affecting digestion.
Cholera:
Cholera is caused by a bacterial infection that disrupts Cl- transport in intestinal cells.
The loss of Cl- ions leads to excessive water loss through diarrhea, which can be life-threatening if not treated promptly.
People with CF, who already have defective CTFR channels, are often resistant to cholera infections. This highlights the complex interplay between genetic factors and disease susceptibility.