BioPharm Final Totonchy
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
Look up a list of monoclonal antibody therapeutics and practice determining the type (mouse, chimeric, humanized, human) based on the drug name. (old naming conventions).
Cetuximab
-XIMAB → chimeric
Natalizumab
-ZUMAB → humanized
Panitumumab
-UMAB → human
Ibritumomab
-MOMAB → mouse (murine)
What factors make mAb therapeutics more or less likely to elicit anti-therapeutic immune responses?
Fully human mABs are less likely vs humanized < chimeric < murine
What are the advantages and drawbacks of nanobodies as therapeutics compared to mammalian immunoglobulins?
Advantages: Ease of manufacture, advantage of stability, one protein dictates the binding domain rather than two
Drawbacks: Very small, still working on BA for these
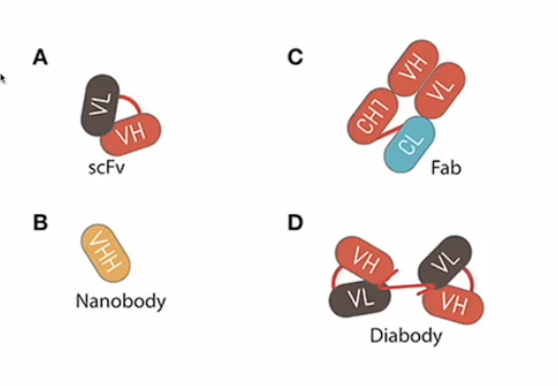
Which mAB structures have lowest BA and need mods to improve efficacy? * Not on study guide on POLL
Based on size so the smaller ones
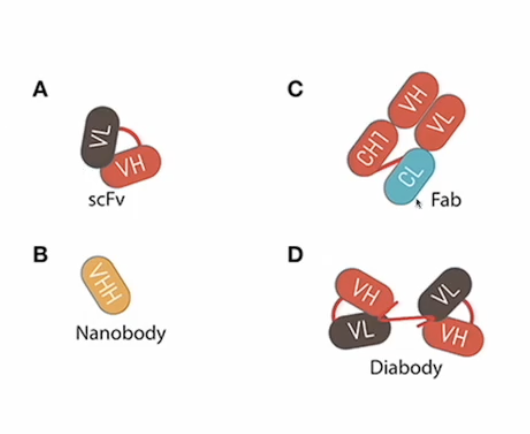
Which mAB structure can be used to crosslink 2 different proteins? *POLLEV
The IgG like bispecific antibody
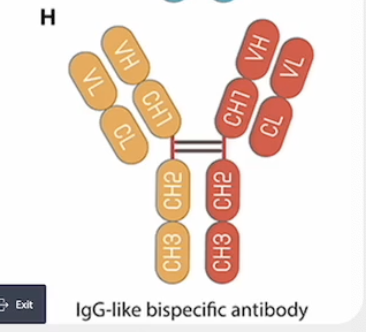
Which applications of mAb therapeutics require the Fc region? (make sure you know what an antibody with an Fc region looks like)
Destroying a target cell based on expression of a single protein
In the picture it is F or G because E does not have Fc and H is for 2 different proteins
**Fc region is crucial for mediating immune effector functions — causing the immune system to destroy a target cell
Antibody-dependent cell-mediated cytotoxicity (ADCC):
NK cells recognize the constant region (Fc) of the antibodies
if you want the therapeutic antibody to target cells for destruction using an ADCC mechanism, it has to have an Fc
if you want a response, the Fc region should match the species
if Fc is NOT human → NO ADCC bc human NK cells are not going to recognize it
Antibody-dependent cellular phagocytosis (ADCP):
Fc engages with Fc receptors on macrophages or dendritic cells to promote phagocytosis of the target.
Complement-dependent cytotoxicity (CDC):
Fc interacts with complement proteins to activate the complement cascade, leading to cell lysis
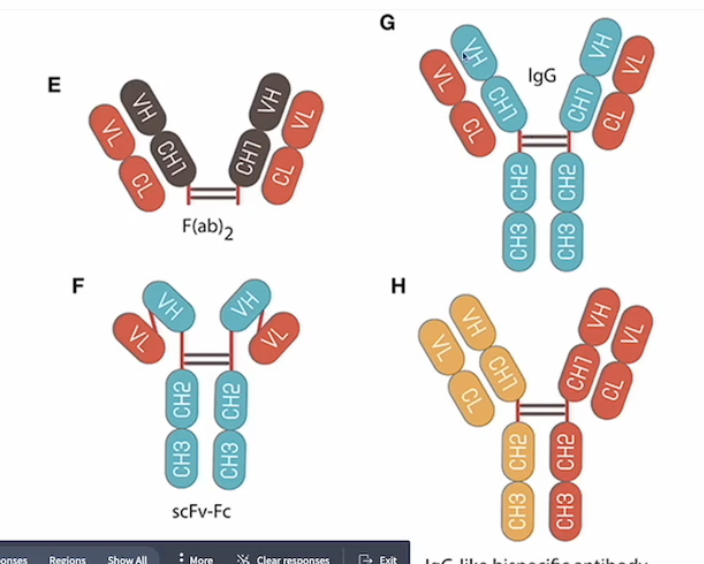
Which applications require two antigen binding sites and what are these antibodies called?
Crosslinking
facilitates crosslinking of antigens, which is critical in signaling or neutralization (e.g. anti-CD20 therapies like rituximab)
Enhanced Avidity
simultaneously bind to two identical epitopes, increasing binding strength and their therapeutic efficacy
Antibodies are called bispecific antibodies
generated by utilizing protein engineering techniques to link two antigen binding domains (such as Fabs or scFvs), allowing a single antibody to simultaneously bind different antigens
Thus, bispecific antibodies may be engineered to exhibit novel functions, which do not exist in mixtures of the two parental antibodies
Most bispecific antibodies are designed to recruit cytotoxic effector cells of the immune system to target pathogenic cells
Which applications require a small structure like a fragment?
Smaller antibody structures such as Fab fragments, scFv, or nanobodies are utilized when:
Rapid tissue penetration is necessary (e.g., Ranibizumab for macular degeneration)
Minimal immune engagement is required to avoid immune activation
Specific targeting in narrow spaces or crossing the blood-brain barrier (e.g., nanobody-based therapie
Carrying a cytotoxic drug into a solid tumor
What terms are used for therapeutics that use the patient’s own cells? What terms are used for therapeutics that use other people’s cells?
Autogenic (Autologous) = patient’s own cells
Allogenic (Heterologous) = someone else’s cells
What factors limit development of CAR T cell therapeutics (hint: mAb also have this limiting factor)? What toxicities are common with CAR T cell therapy?
You need a defined protein target to rationally design a CAR T cell that will seek and destroy a specific target
Toxicities:
On target, off tumor toxicities (ex: normal B cells also express CD19)
Systemic cytokinę toxicities: a result of activated/proliferating CAR T cells
What are the three approaches for gene therapy and how do they work?
Gene therapy is designed to reduce the effect of a mutated gene
Three approaches:
Reduce/prevent the expression of protein from a mutated gene (gene silencing)
Pros: gets rid of overactive genes that are defective, safe (no alteration of genome)
Cons: temporary, continuous therapy
Fix the mutated gene (genome editing)
Pros: will fix any problem
Cons: dangerous (off target mutation)
Add in a good copy of the mutated gene (ectopic expression) ONLY FDA APPROVED
Pros: long term, safe
Cons: uses viral vectors which can be dangerous to some, only specific applications are long term
Which three types of viruses are currently in use as gene therapy vectors? For each one, is it an episomal or integrating vectors?
AAV (Adeno-associated virus) → episomal
Lentivirus → integrating
HSV1 STAR-D → episomal
How do episomal and integrating gene therapy vectors differ? What are safety concerns for each? What are therapeutic durability concerns for each?
Episomal vector:
Poor persistence but safer
Vector immunity can be an issue with repeated therapy (bc the body starts to recognize it more)
Episomal AAV: good long term maintenance in non-dividing cells but questionable maintenance in dividing cells, vector immunity can be an issue
Integrating gene vector:
persistence, more durable, but dangerous because you might integrate a tumor suppressor gene and cause cancer
Integrates directly into the host genome and gets replicated as cells divide
Hard to control where it goes or functions
Integrating lentivirus: both autologous cell based and gene therapy, tel-bone marrow transplant except stem cells are fixed before being admin
What is the only FDA approved oncolytic therapeutic?
Imlygic (Talimogene Laherparepvec)
The ethical and practical reasons behind placebo choice for vaccine clinical trials.
If we need to mimic reactogenicity (AE), we use a non-inactive placebo to preserve blinding of the subjects, this is important if risk behaviors affect a subject’s risk for disease in an efficacy endpoint study
In addition, if we have an established standard of care (in the same target group, meaning if there is already an effective and approved vaccine, using an inactive placebo denies a pt to tx that is already safe), then an inactive placebo IS NOT ethical
Which vaccine types require specific target antigens to be selected during development?
Adjuvants subunit
mRNA/DNA