States of Matter, States of Matter
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
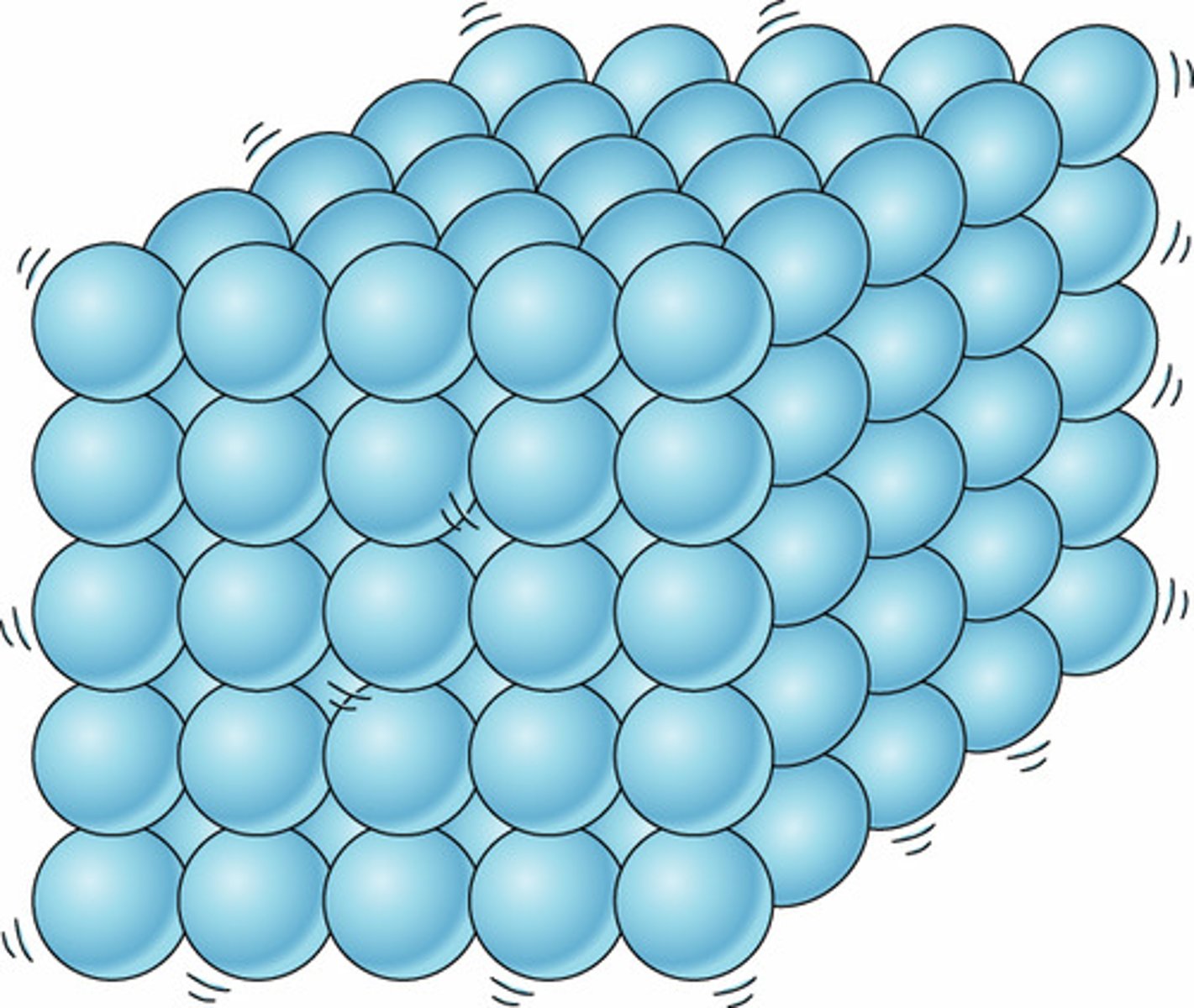
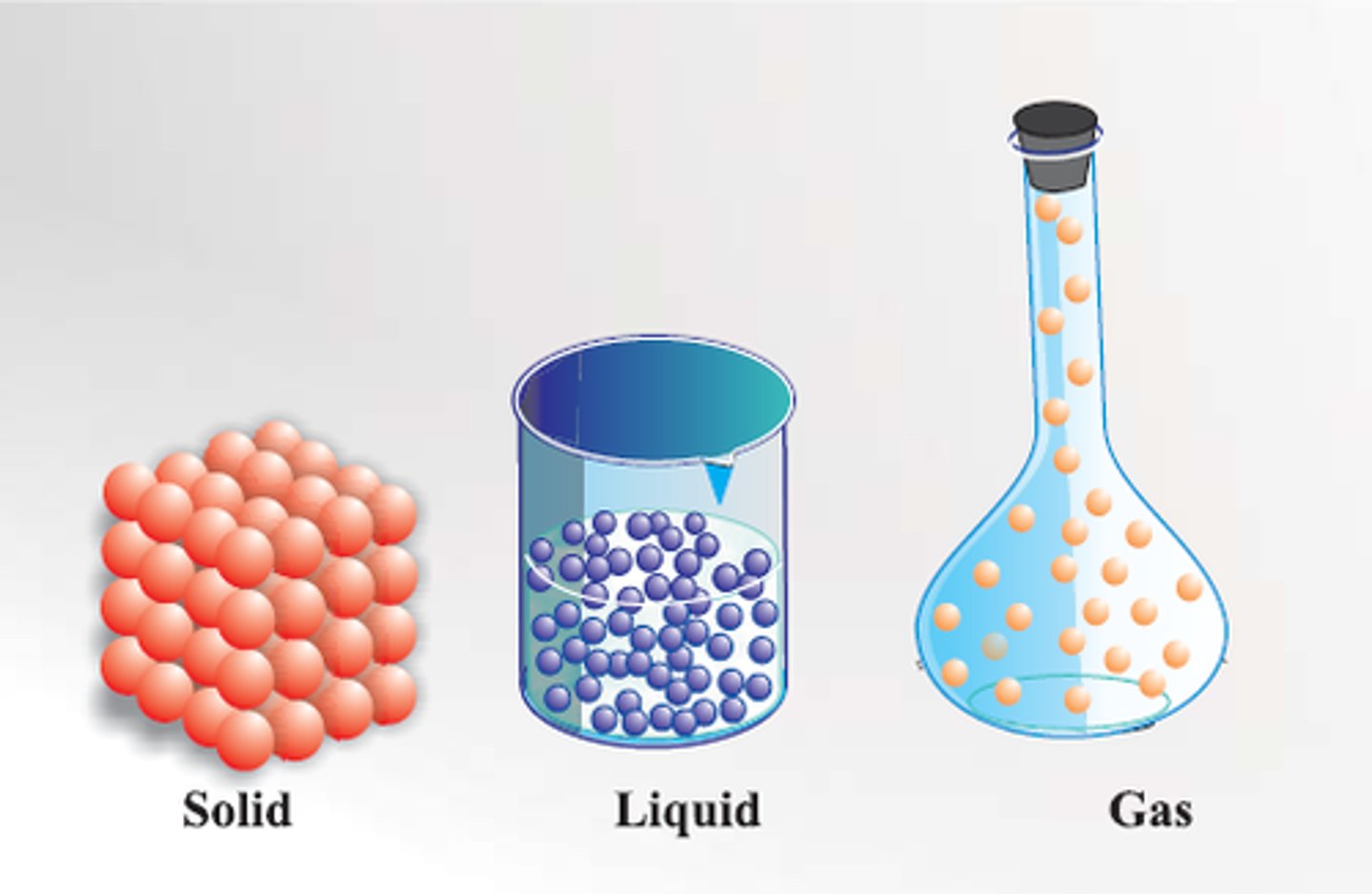
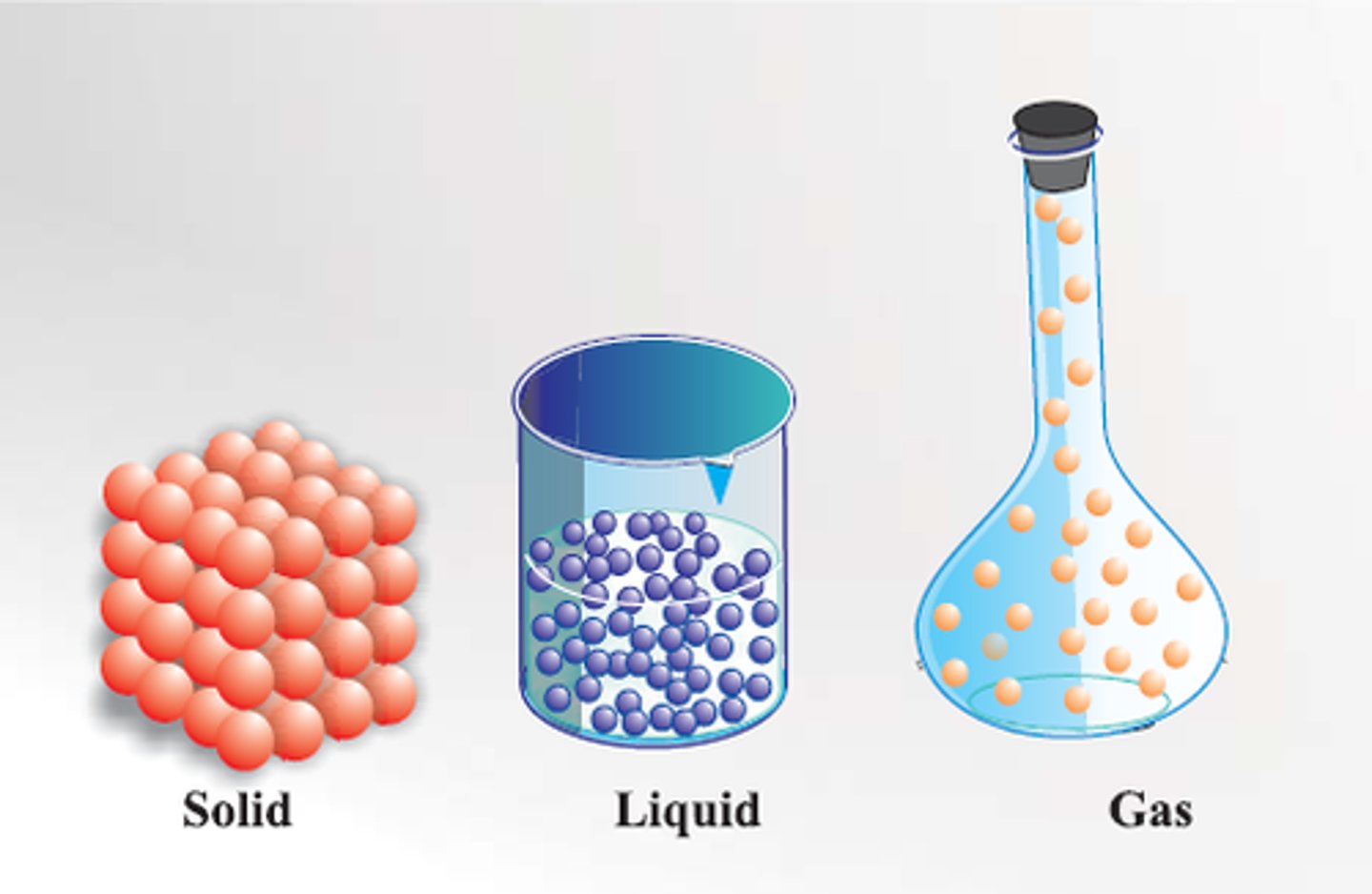
solid
The particles are tightly packed.
The particles do not move around.
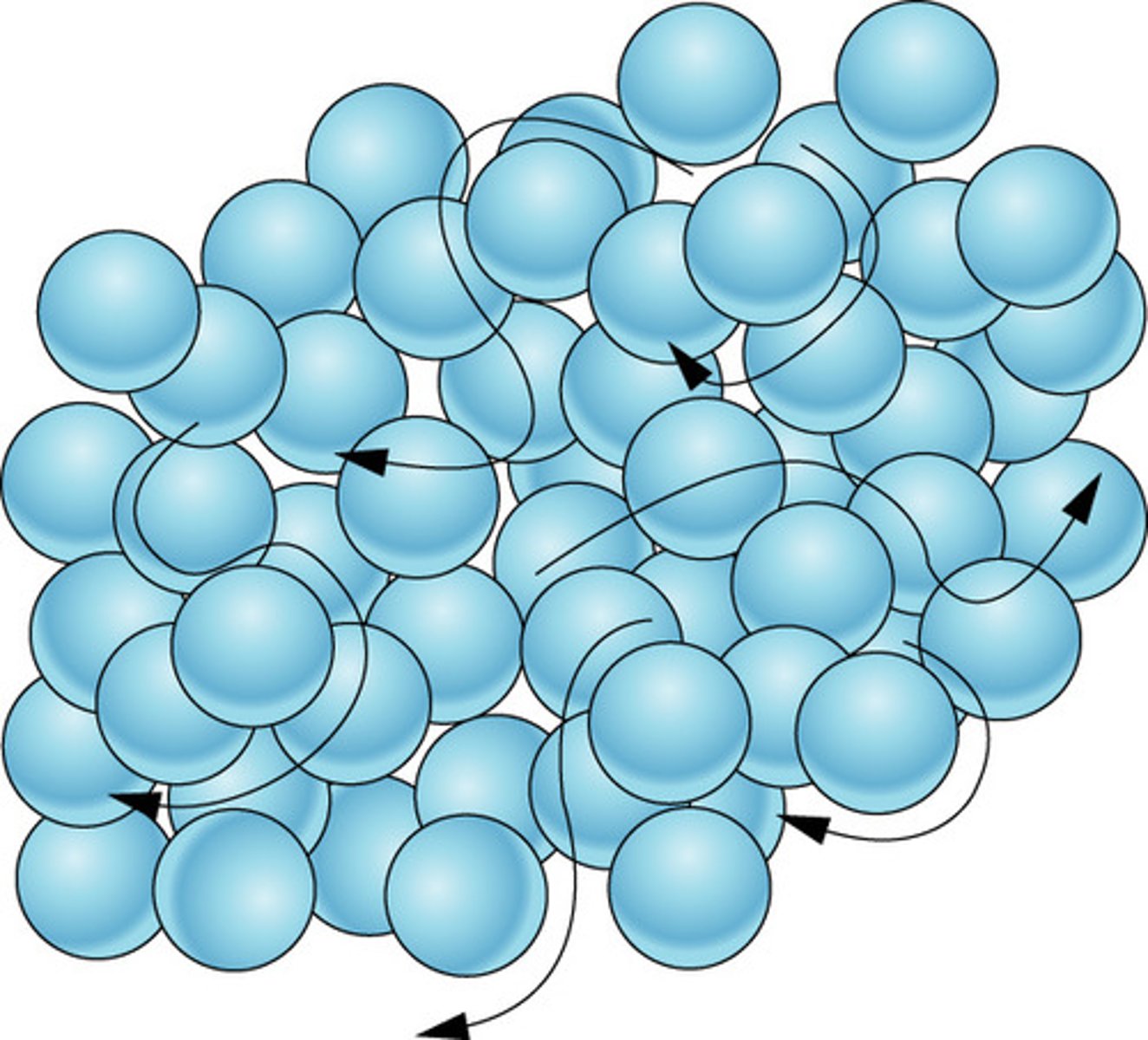
liquid
The particles are close together.
The particles move, and slide past each other.
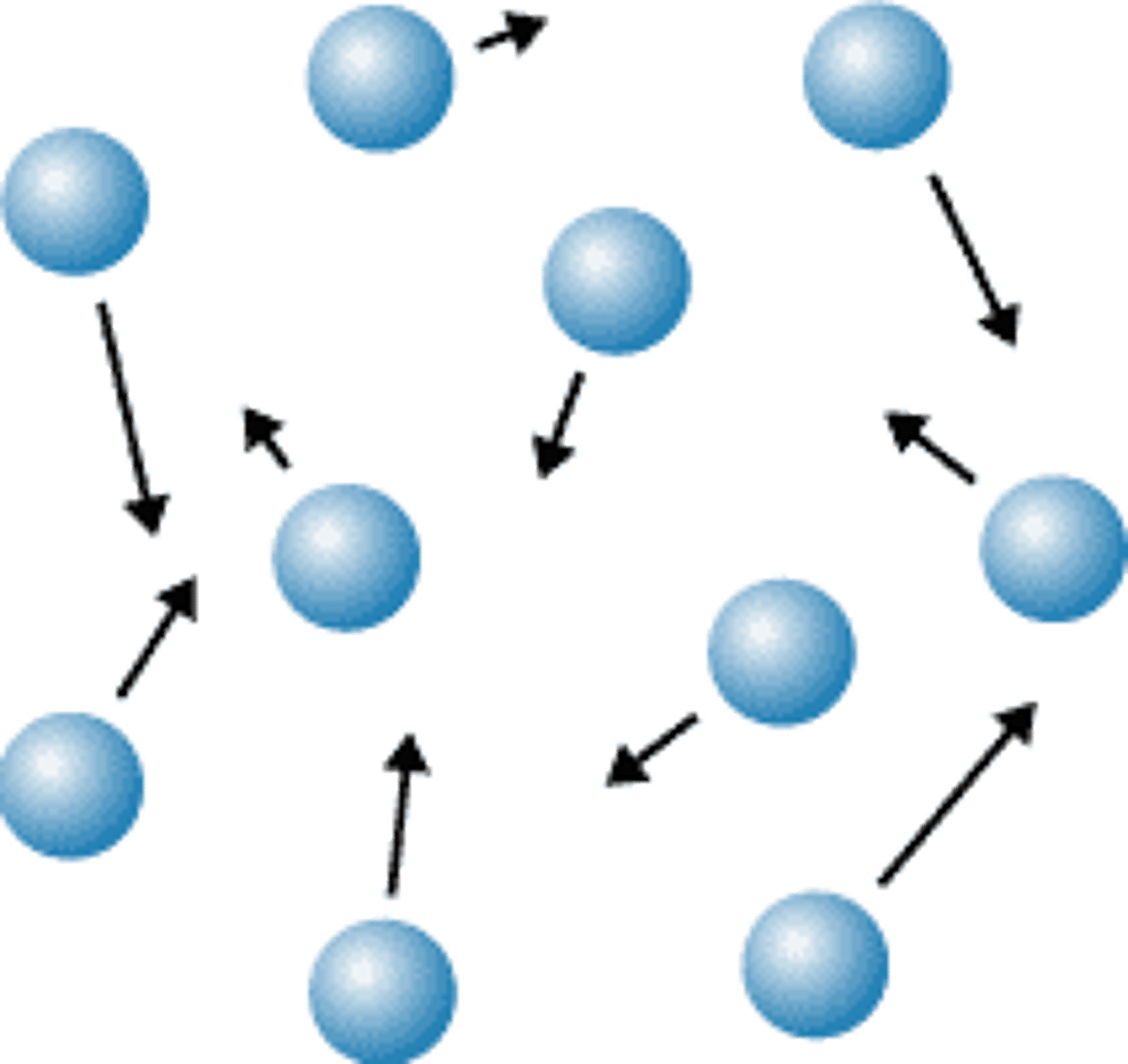
gas
The particles are very separated.
The particles move quickly.
condensation
Gas to liquid:
(Dew on grass)

evaporation
Liquid to Gas:
(Puddles disappearing)

melting point
Solid to liquid:
(Ice to water)

freezing point
Liquid to solid:
(Water to ice)

Liquid
can be poured and take on the shape of their container. Has mass and volume.

Evaporation
the change of a liquid to a gas

Matter
anything that takes up space and has mass
Particles
Tiny atoms or molecules that make up all matter
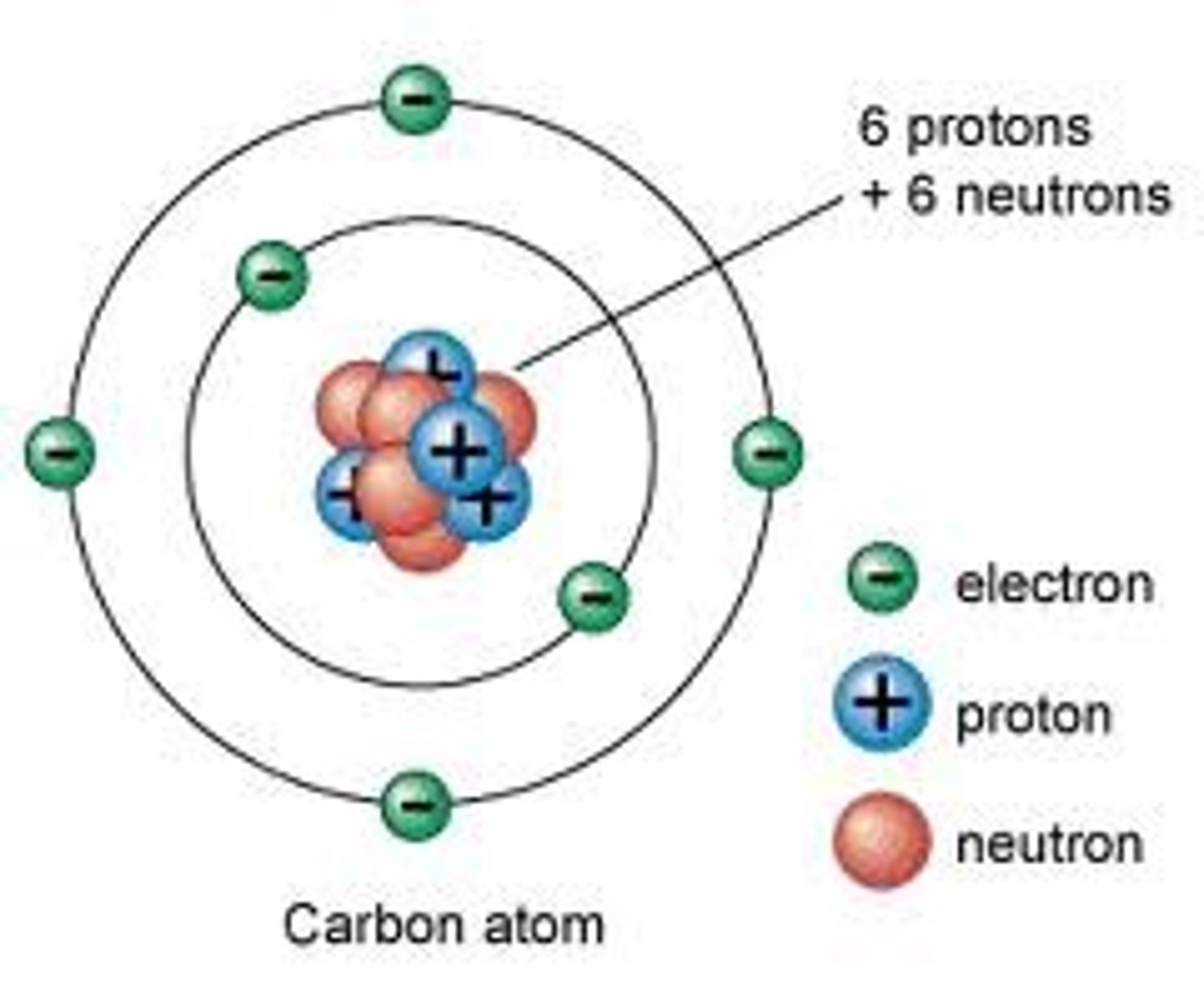
atoms
basic particle from which all elements are made
element
cannot be broken down into any other substance
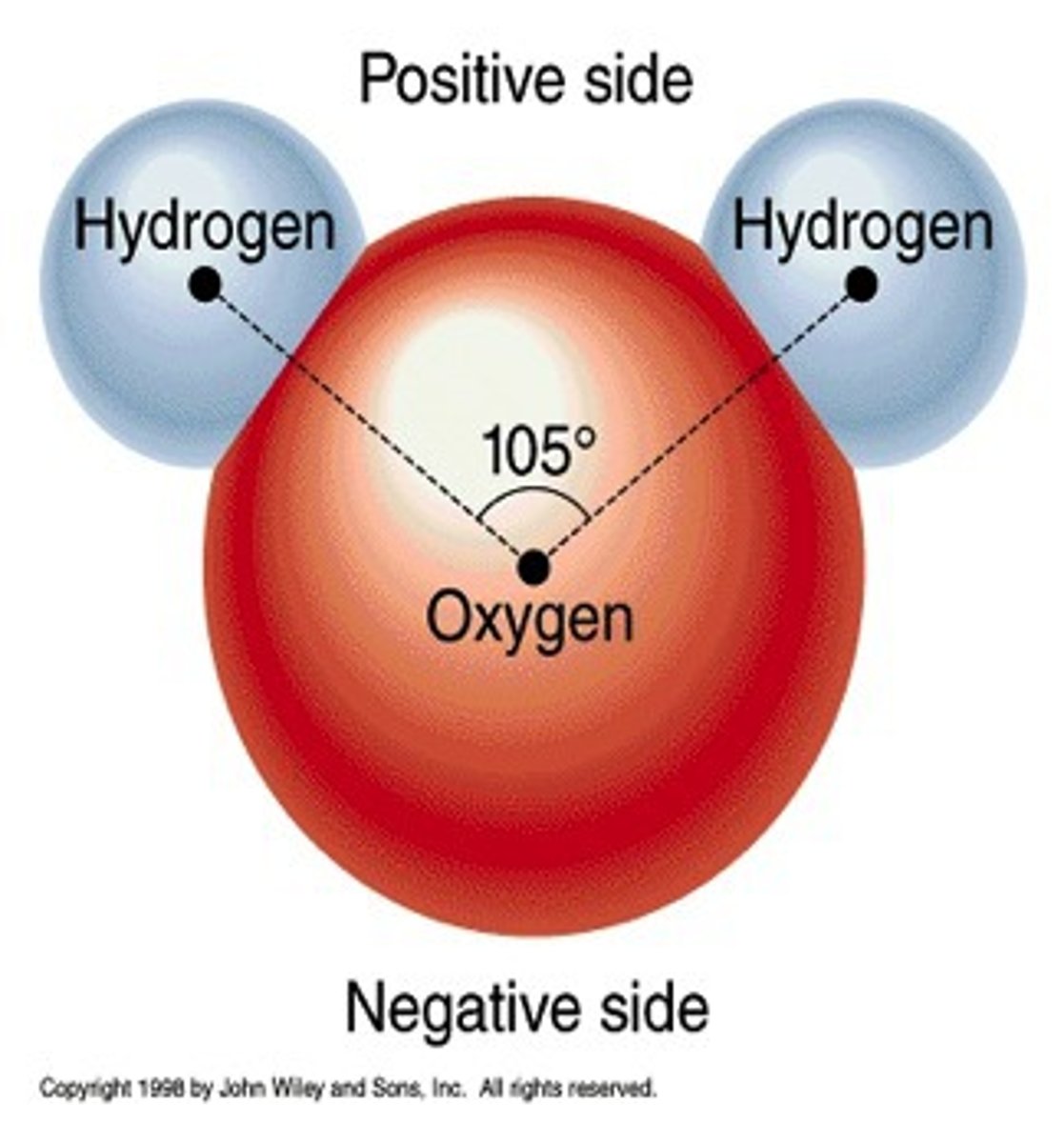
molecule
2 or more atoms bonded (same or different)
matter
anything that has mass and takes up space
physical change
a change of matter from one form to another without a change in chemical properties
chemical change
A change in matter that produces one or more new substances
A car rusting is an example of what type of change?
chemical change
liquid water to water vapor (gas)
physical change
Density
Mass/Volume
Mass
density x volume
Volume
The amount of space an object takes up
Sublimation
the process in which a solid changes directly into a gas
heat energy
a form of energy that is transferred by a difference in temperature
Temperature
A measure of the average energy of motion of the particles of a substance.
conduction
Form of heat transfer where heat energy is directly transferred between molecules through molecular collisions or direct contact.
water vapor
water in the form of a gas
adhesion
force of attraction between different kinds of molecules
Cohesion
Attraction between molecules of the same substance
molecular motion
the movement of molecules in matter