Unit 2 Topic 6: Resonance & Formal Charge
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Last updated 7:03 PM on 11/30/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
1
New cards
Resonance structures
2 or more lewis electron-dot structures which both represent correct structures for a molecule
2
New cards
Formal Charge
A way of assigning a charge to each individual atom in a molecule; in a neutral molecule the most stable condition for each atom is to have a formal charge of zero
3
New cards
Formal charge formula
[# of valence electrons of an element] - [# of assigned valence electrons to the element in the lewis-dot structure] = formal charge
Each dot count as one and bond count as one
4
New cards
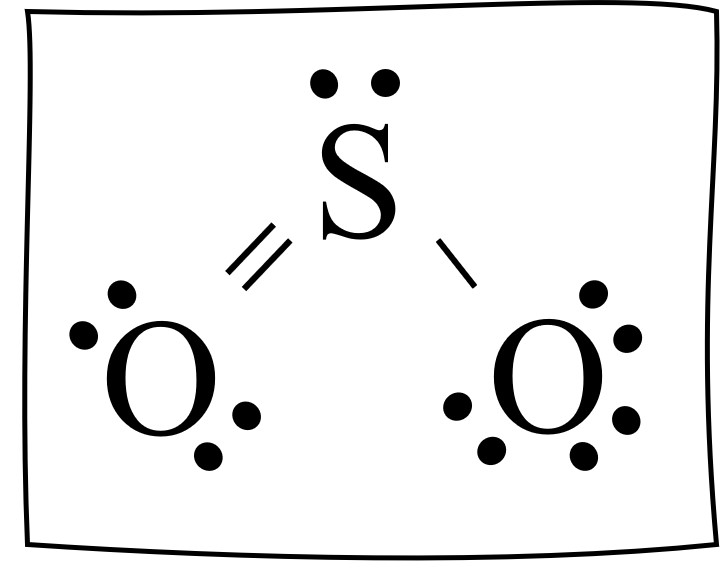
Find the formal charge of SO2
Oxygen on the left: 6- 6 = 0
Sulfur: 6 - 5 = 1
Oxygen on the right: 6-7 = -1