PH Scale, PH Formula, Titration Formula, Error Propogation, and Uncertainty Formula
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
pH scale
The pH scale expresses the strength of an acid or base.
It measures [ H3O+ ] or [H+] (the hydrogen ion concentration.)
The pH scale ranges from 0 – 14.
pH < 7 is acidic
pH > 7 is basic
pH = 7 is neutral
A pH of 7.3 is slightly basic.
Only exactly 7 is neutral.
![<ul><li><p><span>The pH scale expresses the <strong><u>strength</u></strong> of an acid or base.</span></p><ul><li><p><span>It measures [ H<sub>3</sub>O<sup>+</sup> ] or [H<sup>+</sup>] (the <strong><u>hydrogen ion</u></strong> concentration.)</span></p></li></ul></li><li><p><span>The pH scale ranges from 0 – 14.</span></p><ul><li><p><span>pH < 7 is <strong><u>acidic</u></strong></span></p></li><li><p><span>pH > 7 is <strong><u>basic</u></strong></span></p></li><li><p><span>pH = 7 is <strong><u>neutral</u></strong></span></p><ul><li><p><span>A pH of 7.3 is slightly basic.</span></p></li><li><p><span>Only exactly 7 is neutral.</span></p></li></ul></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/b9c27c20-77e5-40e8-8ce2-957196e6d878.png)
The pOH Scale
The pOH is the REVERSE of the pH scale!
pOH tells you how basic a solution is
Scale from 14 to 0
The lower the pOH, the more basic the solution.
The higher the pOH, the more acidic the solution.
Never use pOH to figure out if something is acidic or basic! ALWAYS convert to pH!
pH and pOH Formulas
pOH + pH = 14
How do we calculate how acidic or basic a solution is?
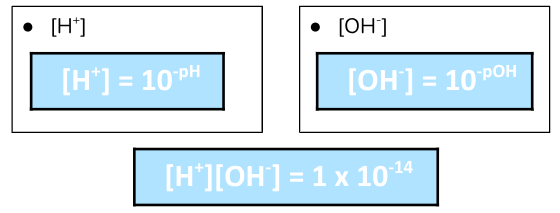
pH measures the concentration of hydrogen ions [H+]
pOH measures the concentration of hydroxide ions [OH-]
![<ul><li><p><span><strong><u>pH</u></strong> measures the concentration of hydrogen ions <strong>[H<sup>+</sup>]</strong></span></p></li><li><p><span><strong><u>pOH</u></strong> measures the concentration of hydroxide ions <strong>[OH<sup>-</sup>]</strong></span></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/b67e2abd-8f2a-44be-ba8e-05c2083ba1d1.png)
Calculating [H+] or [OH-]
To solve for the concentration of H+ or OH-, we use antilog!
Titration
An experimental technique used to determine the concentration of an unknown solution.
This is done by using a known concentration of one substance and adding it to another solution of an unknown concentration.
Titration Calculations
We use the same equation for titrations as for dilutions:
M1V1 = M2V2
For titrations, we’re solving for the volumes at which the moles of acid equal the moles of base present.
Types of Experimental Errors
Random
Can go in either direction
Typically from instrumentation
Give us ± value
Systemic
Go in a particular direction
Flaw in instrument
Fix with calibration
Flaw in method
Separation lab – mass would be too high if sand or salt is still wet
Uncertainty in Calculations- Addition & Subtraction
When doing a calculation that involves adding or subtracting, add absolute uncertainties directly from instrument
Ex:
1.2 cm ± 0.1 cm
+2.5 cm ± 0.1 cm
3.7 cm ± 0.2 cm
Uncertainty in Calculations- Multiplication & Division
When doing a calculation that involves multiplying and/or dividing
Find the percent uncertainty of each measurement
(uncertainty ÷ measurement) * 100
Add all of the percent uncertainties together
Value can be reported as percent.
Density = m/V
mass = 4.35 g ± 0.2% = 0.78 g/ml ± 2%
Volume = 5.6 mL ± 2%
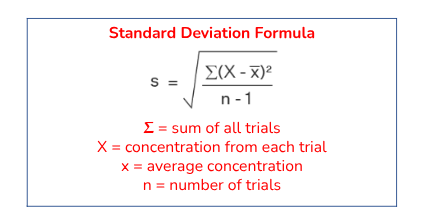
Uncertainty in Calculations- Averages
When doing the average of calculated values, standard deviation is used for propagation of error