Ionisation energy
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
What is ionisation energy?
the minimum energy required to lose one mole of electrons from one mole of gaseous atoms in the ground state
Define the term first ionisation energy
The amount o energy required to remove one mole of gaseous atoms to form one mole of gaseous ions each with a charge of 1+
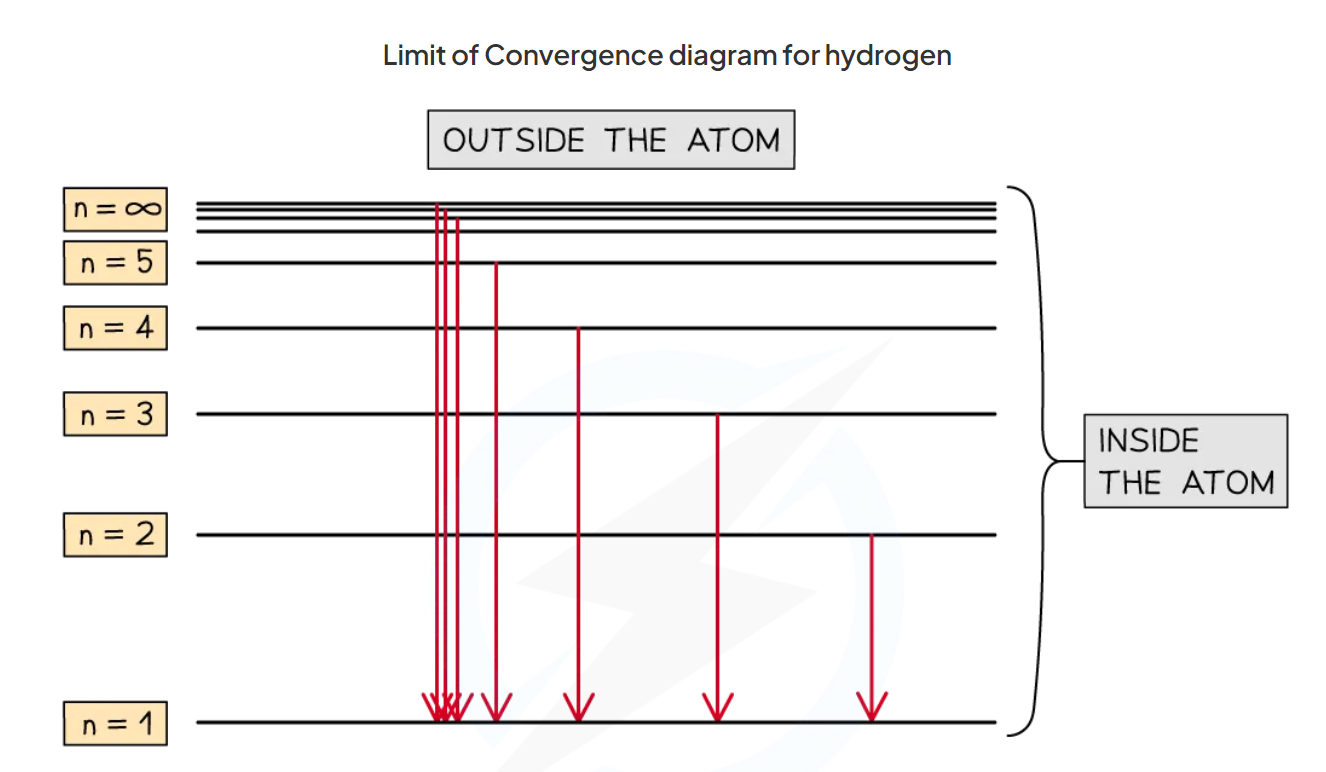
What is the limit of convergence?
the frequency at which the spectral lines converge
(Convergence: where the lines become so close that it is indistinguishable)
The frequency or wavelength that corresponds to ionisation
What is required to overcome the type of attraction to loose an electron?
energy is required to overcome the electrostatic attraction between the positively charged nucleus and the negatively charged electrons.
What factors effect the first ionisation energy?
Size of the Nuclear Charge:
Distance of Outer Electrons from the Nucleus:
Shielding Effect of Inner Electrons:
Spin-Pair Repulsion:
How does the size of the nuclear charge effect first ionisation energy?
An increase in the nuclear charge (more protons in the nucleus) leads to a stronger electrostatic attraction between the positively charged nucleus and negatively charged electrons.
This stronger attraction makes it more difficult to remove an electron from the atom.
As a result, an increase in the nuclear charge generally leads to a higher first ionization energy.
How does the distance of electrons from the nucleus effect first ionisation energy
Electrons in outer energy levels are farther from the nucleus, and they experience a weaker attractive force.
This weaker attraction makes it easier to remove an outer electron, resulting in a lower first ionization energy for atoms with outer electrons located farther from the nucleus
How does spin pair repulsion effect the first ionisation energy?
Electrons must have opposite spins when occupying the same orbital, which leads to repulsion between them.
This repulsion causes a slight decrease in the first ionization energy because it requires less energy to remove an electron that experiences spin-pair repulsion.
Describe the trend in the first ionisation energy down a group.
Going down a group the first ionisation energy decreases.
Going down a group atomic radii increases and electrons occupy higher energy levels.
Electrons in higher energy levels have a weaker attraction to the nucleus.
Less energy is required in order to remove an electron.
Describe the trend in first ionisation energy going across a period.
Going across a period the first ionisation energy increases.
Going across a period there are more protons in the nucleus and more electrons occupying the outermost energy level (increasing effective nuclear charge).
A greater number of electrons in outermost energy level results in a stronger attraction to the nucleus.
More energy is required in order to remove an electron.
The ionisation of an atom
At this point the electron has been removed from the attraction of the nucleus and the atom has been ionised.
n = 1 to n = ∞
How can the emission spectrum of an element be used to find the first ionisation energy of its outermost electron?
By determining the energy of the convergence limit of the series which relates to transition to the energy level of the outermost electron
Why are data on successive ionisation energies of a transition metal often not useful in assigning its group number?
The successive ionisation energies of transition elements are quite similar, as the outer shell electrons are in the same shell, so there are no large jumps in ionisation energies.
How is the first ionisation energy deduced?
from the convergence limit of the series with the shortest wavelength
Explain why an energy level (such that of hydrogen) consists of a number of different series of lines?
The different series represents different energy levels
What effect does the repulsion between electrons in a doubly occupied orbital have in the ionisation energy?
Repulsion between electrons in a double occupied orbitals decreases the ionisation energy.
Hence electrons in a doubly occupied orbital require less energy to remove.
Successive ionisation energy
refers to the process of removing electrons from an atom or positive ion.
How to deduce the group of an element from its successive ionisation data
when there is a large energy change between two groups of ionisation energy
(for example between group 3 and 4)
i indicates that it is much easier to remove the first three electrons than the fourth.
Hence the electron must be in group 3.
Describe the motions in a successive ionisation graph
The big jumps on the graph show the change of shell and the small jumps are the change of subshell
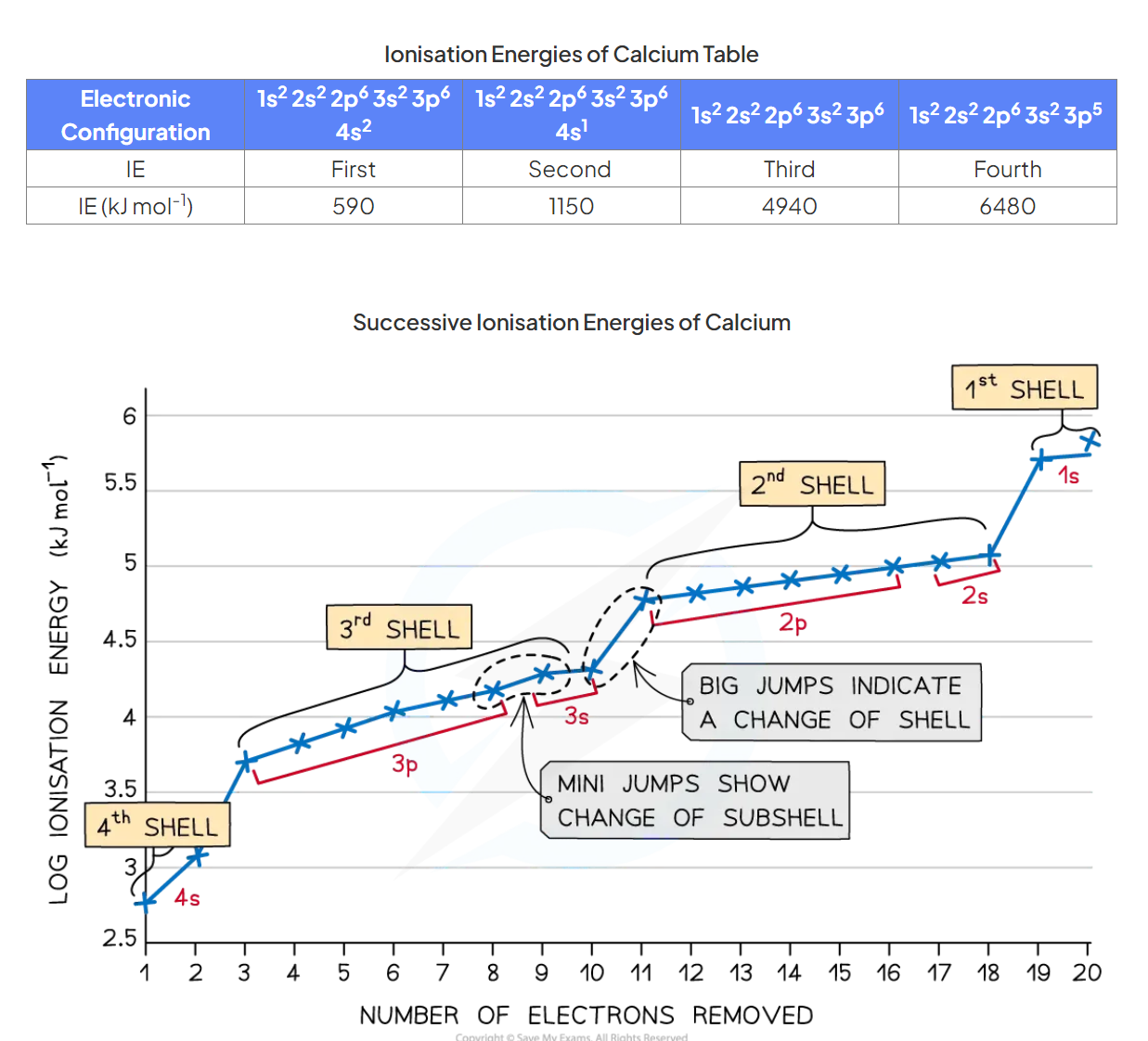
What is the order in which electrons are removed from the shells?
They are removed from the highest shelves first.
f>d>p>s
Explain the trend in successive ionisation energy
It shows an increase. The reason for this is that after the first ionisation, the electrons are being removed from an increasingly positive ion. This leads to an increase in the electrostatic attraction between the nucleus and the remaining electrons and an increase in the ionisation energy.
sucessive ionsisation energy: energy required to remove electrons
The successive ionisation energies of an element increase
This is because once you have removed the outer electron from an atom, you have formed a positive ion
Removing an electron from a positive ion is more difficult than from a neutral atom
As more electrons are removed, the attractive forces increase due to decreasing shielding and an increase in the proton to electron ratio
How to form the equation for ionisation energy? write it for the second ionisation energy of calcium.
Make sure the state symbol is a gas. And the number of ionisation energy determines how many electrons it has lost.
The second ionisation of calcium is the removal of one mole of electrons from the Ca+ ion to form the Ca2+ ion in the gaseous state.
Ca+(g) → Ca2+(g) + e−
In which ionisations would a group 14 element have the largest difference?
An element in group 14 would have the largest difference in ionisation energy between the 4th and 5th ionisations. This is because the fifth electron is removed from an energy level closest to the nucleus and requires more energy to remove.
What factors affect electron removal energy in atoms?
Electrons in higher energy orbitals require less energy to remove than electrons in lower energy orbitals due to their distance from the nucleus.
Electrons in doubly occupied degenerate orbitals require less energy to remove than electrons in singly occupied orbitals due to repulsive forces.
Explain why there is a large decrease in ionisation energy between the last element of one period to the first element of the next period? (Example: He-li or Ne-Na)
There is an increased distance between the nucleus and the outer electron as there is a new shell.
There is Increased shielding by inner electrons because of the added shell
These two factors outweigh the Increased nuclear charge
Explain why there is a slight decrease in ionisation energy between beryllium and boron.
the fifth electron in boron is in the 2p subshell, which is further away from the nucleus than the 2s subshell of beryllium
Beryllium’s electron configuration is 1s2 2s2
Boron’s its electron configuration is 1s2 2s2 2px1
Explain why there is a slight decrease in ionisation energy between nitrogen and oxygen.
due to spin-pair repulsion in the 2px orbital of oxygen
Nitrogen’s electron configuration is 1s2 2s2 2px1 2py1 2pz1
Oxygen’s electron configuration is 1s2 2s2 2px2 2py1 2pz1
In oxygen, there are 2 electrons in the 2px orbital, so the repulsion between those electrons makes it slightly easier for one of those electrons to be removed
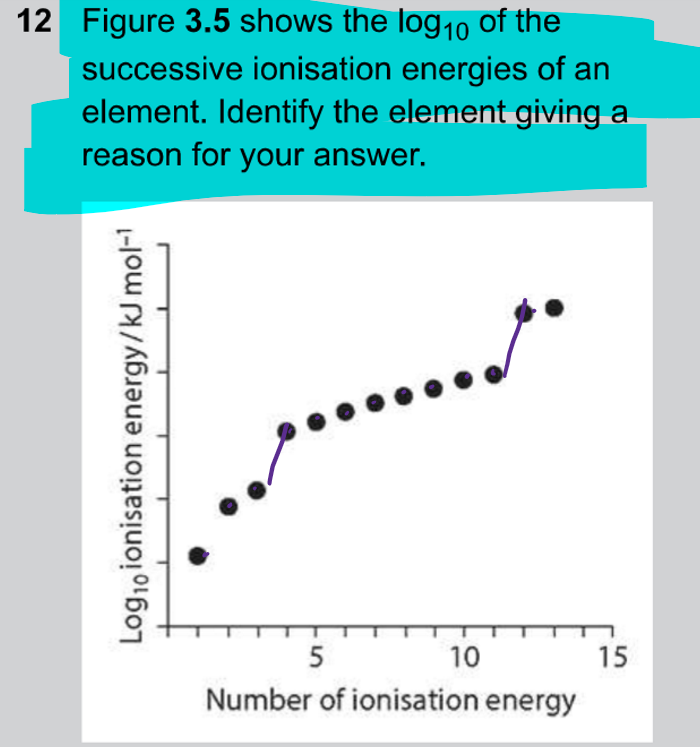
How to answer a question where they ask to deduce the element from its successive ionisation energy?
