AP Chem Unit 3.1-3
1/37
Earn XP
Description and Tags
Mr ionic mentioned??
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
A nonpolar molecule with more electrons is more __________, meaning it has stronger _________
polarizable, LDFs
When molecules are the same size, ______ forces are stronger than _______ forces
dipole-dipole, London disperson
When two molecules have a large difference in size, the strength of their _______ (whether polar or nonpolar) scales with their size
IMFs
Although CCl4 has only LDFs, while HCl has dipole-dipole forces AND LDFs, CCl4 has a stronger IMFs because…
It is much larger than HCl (more electrons)
Hydrogen bonds are really just extra-strong ________ forces
dipole-dipole
What must a molecule have to be a hydrogen bond DONOR?
Hydrogen atom that is covalently bonded to F, O, or N
What must a molecule how to be a hydrogen bond ACCEPTOR?
F, O, or N with a lone pair of electrons on it and a partial negative charge (part of a polar bond)
Hydrogen bond ACCEPTORS don’t need to have…
Hydrogen, just F, O, or N with lone pair
In a large molecule, it is possible for hydrogen bonding forces to be formed between a _____________________ and the _______________
hydrogen atom (bonded to F, O, or N); negative end of a dipole formed by F, O, or N
Ion-dipole forces
Attractive force between ion and polar molecule, usually occurring in a solution
Ion-dipole forces occur when an ionic compound is ___________ in a polar _________, such as…
dissolved, solvent, H2O
Dipole-induced dipole forces
Attractive force between polar and nonpolar molecules → polarity of polar molecule causes a nonpolar molecule to form a temporary dipole
Dipole-induced dipole forced increase with the __________ of the polar molecule and the ____________ of the nonpolar molecule
magnitude, polarizability
Polarizability
The case with which the electron cloud in an atom or molecule can be distorted (larger electron cloud = more polarizable)
London dispersion forces
Attractions between nonpolar atoms/molecules that are caused by temporary dipoles
Dipole-dipole forces
Attractions between polar molecules in which the partial negative charge of one polar molecule is attracted to the positive partial charge on another nearby molecule
If two compounds have the same formula, but one structure has a larger surface area, it will have __________ IMFs
stronger
Amorphous network solid
Solid with a random, disordered arrangement of atoms or molecules
Crystalline network solid
Three-dimensional, repeating arrangement of atoms, ions, or molecules
Ionic and covalent network solids have ________ melting points, while molecular solids have _______ melting points
high, low
_____ solids are hard and brittle
Ionic
_____ solids are hard and rigid
Covalent network
_____ solids are soft
Molecular
_____ solids are malleable and ductile
Metallic
Which is the only SOLID that conducts electricity?
Metallic
Metalloids form _________ bonds with nonmetals
covalent
A higher vapor pressure implies many _________
collisions
Vapor pressure
Pressure exerted by a gas in equilibrium with its liquid phase at a given temperature
Network solids only have _________ and lack ________, giving them ridiculously high _______________
bonds, IMFs, boiling points
When comparing two liquids at the same temperature, the liquid that has a higher ____________ should have a lower ____________
vapor pressure, boiling point
Boiling point
Temperature which the vapor pressure of a liquid is equal to the external pressure surrounding the liquid
Normal boiling point
Situation in which the vapor pressure of a liquid is equal to the standard atmospheric pressure at sea level (1 atm/760 torr)
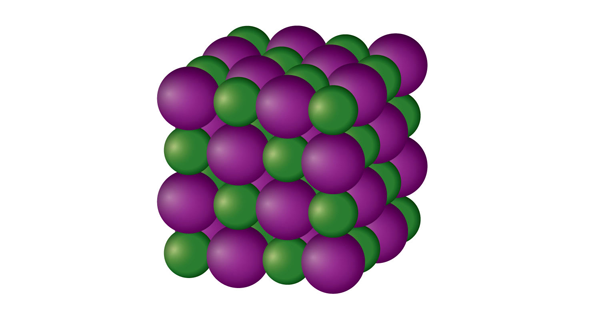
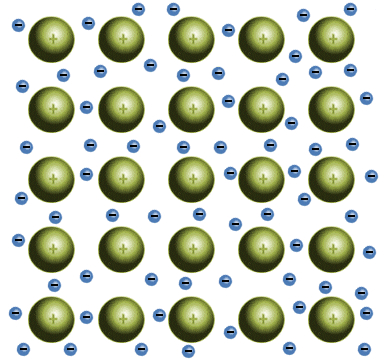
Which solid is represented by the particles?
Ionic
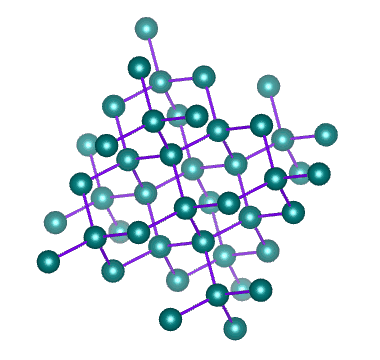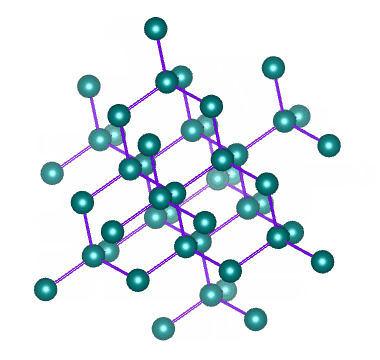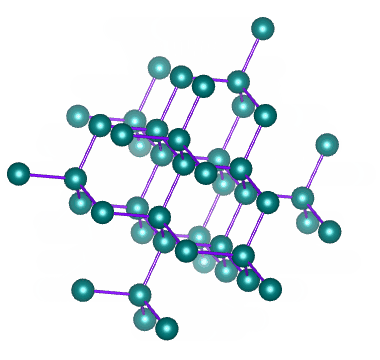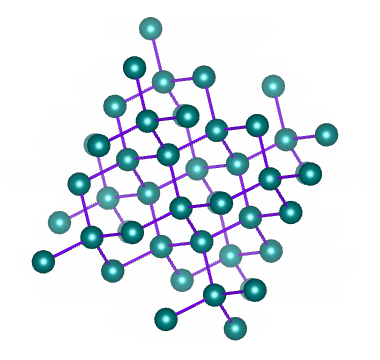
Which solid is represented by the particles?
Covalent network
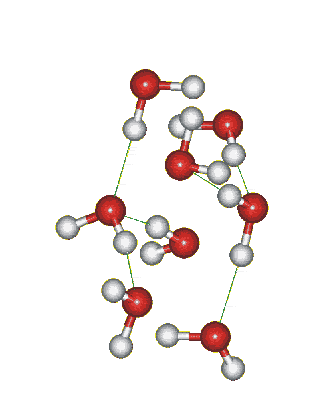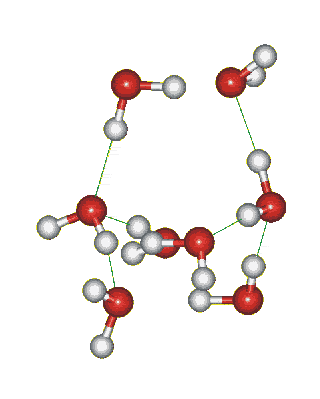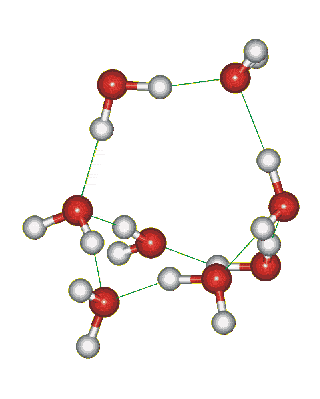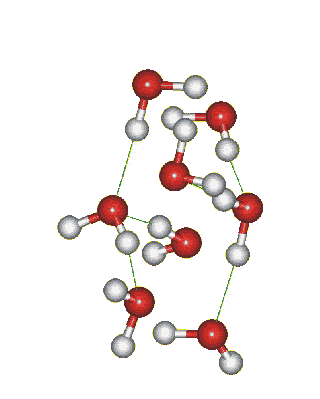
Which solid is represented by the particles?
Molecular
Which solid is represented by the particles?
Metallic
Does Nick Forest know what polarizability is?
No lol
Is Mr Ionic the goat?
[[YES]]
![<p>[[YES]]</p>](https://knowt-user-attachments.s3.amazonaws.com/a7d4cfe8-c0d4-4e3e-b511-9af8a7a0da12.png)