Molecular Geometry
1/11
Earn XP
Description and Tags
AP CHEM
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
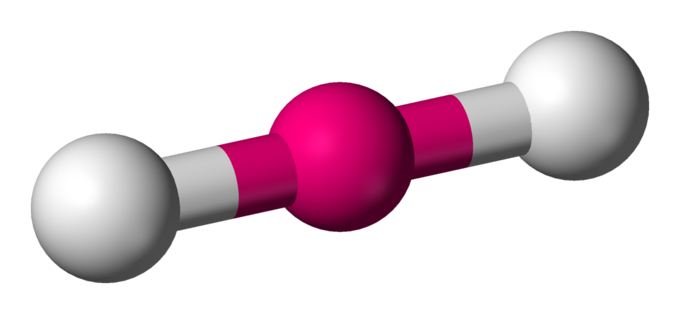
Linear
2 atoms attached to centre molecule
180 degrees
0 lone pairs
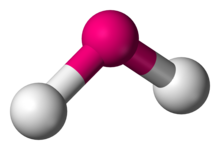
Bent
2 atoms attached to center
<120 degrees
1 lone pair
Bent, «109.5
2 atoms on center
2 lone pairs
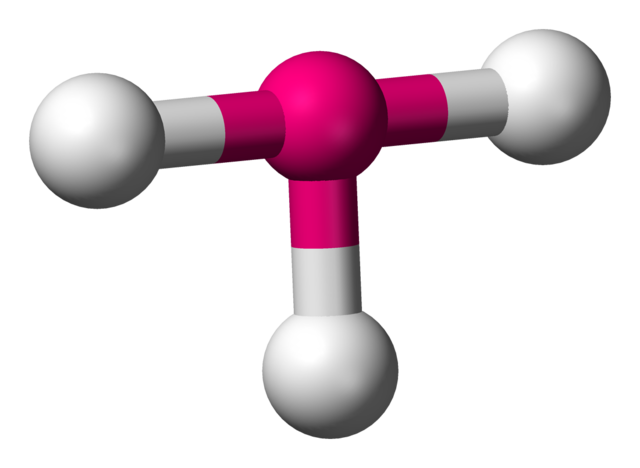
Trigonal planar
3 atoms on center
120 degrees
0 lone pairs
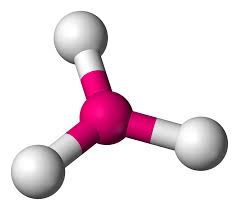
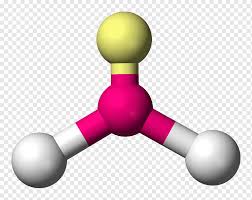
Trigonal pyramidal
3 atoms on center
<109.5 degrees
1 lone pair
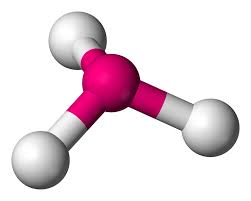
T-shaped
3 atoms on center
< 90 degrees
2 lone pairs
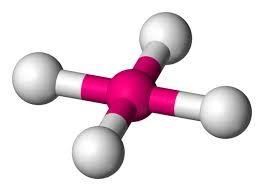
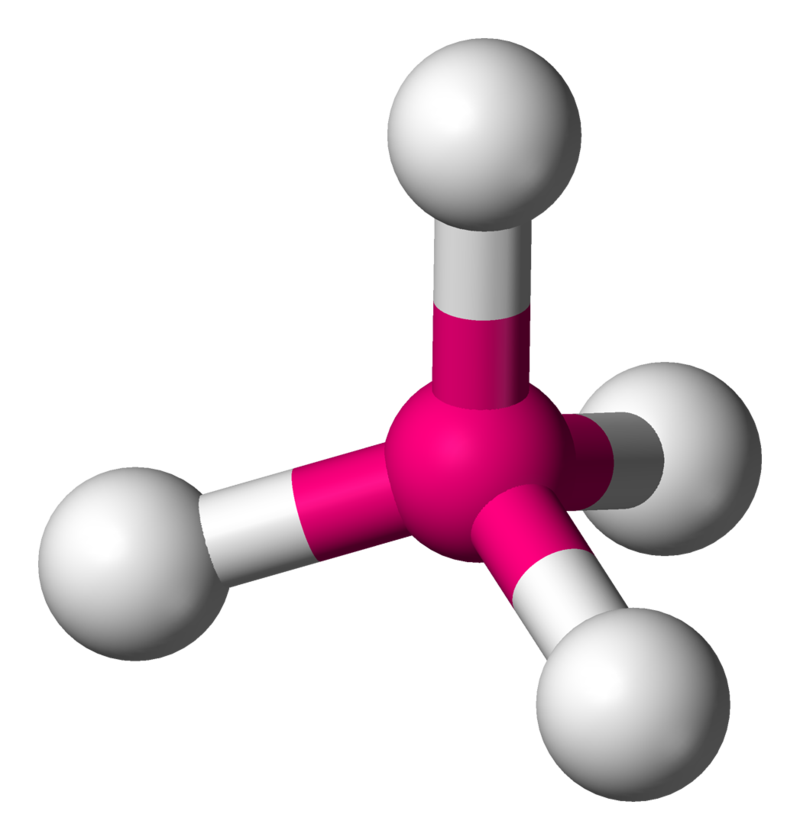
Tetrahedral
4 atoms on center
109.5 degrees
0 lone pairs
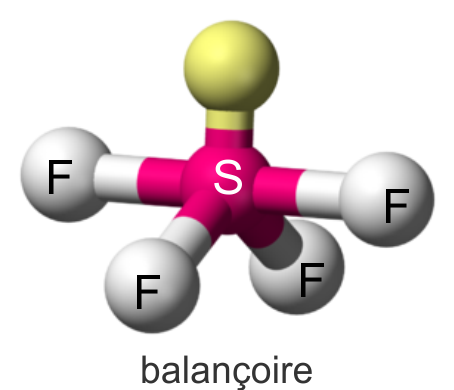
Seesaw
4 atoms on center
<120 and <90 degrees
1 lone pair
Trigonal bipyramidal
5 atoms on center
120 and 90 degrees
0 lone pairs
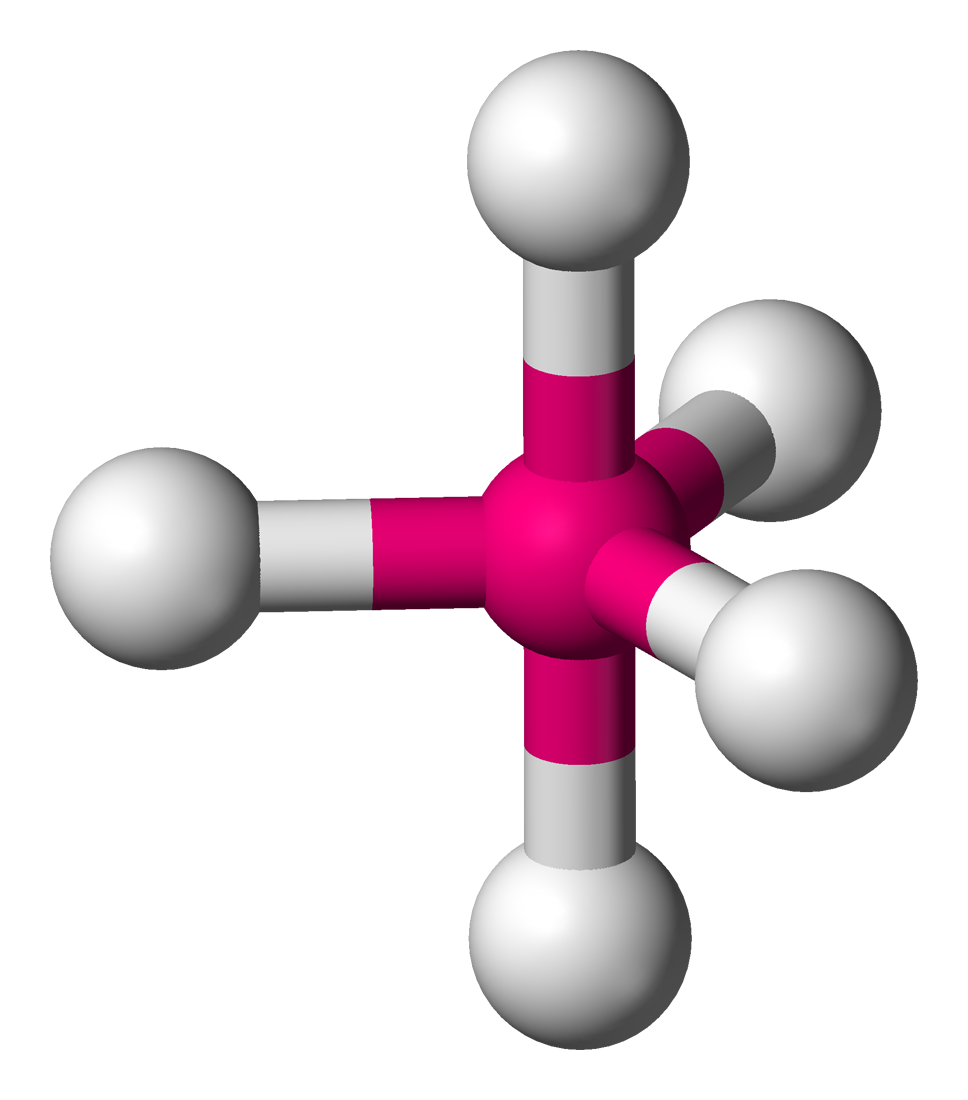
Octahedral
6 atoms on center
90 degrees
0 lone pairs
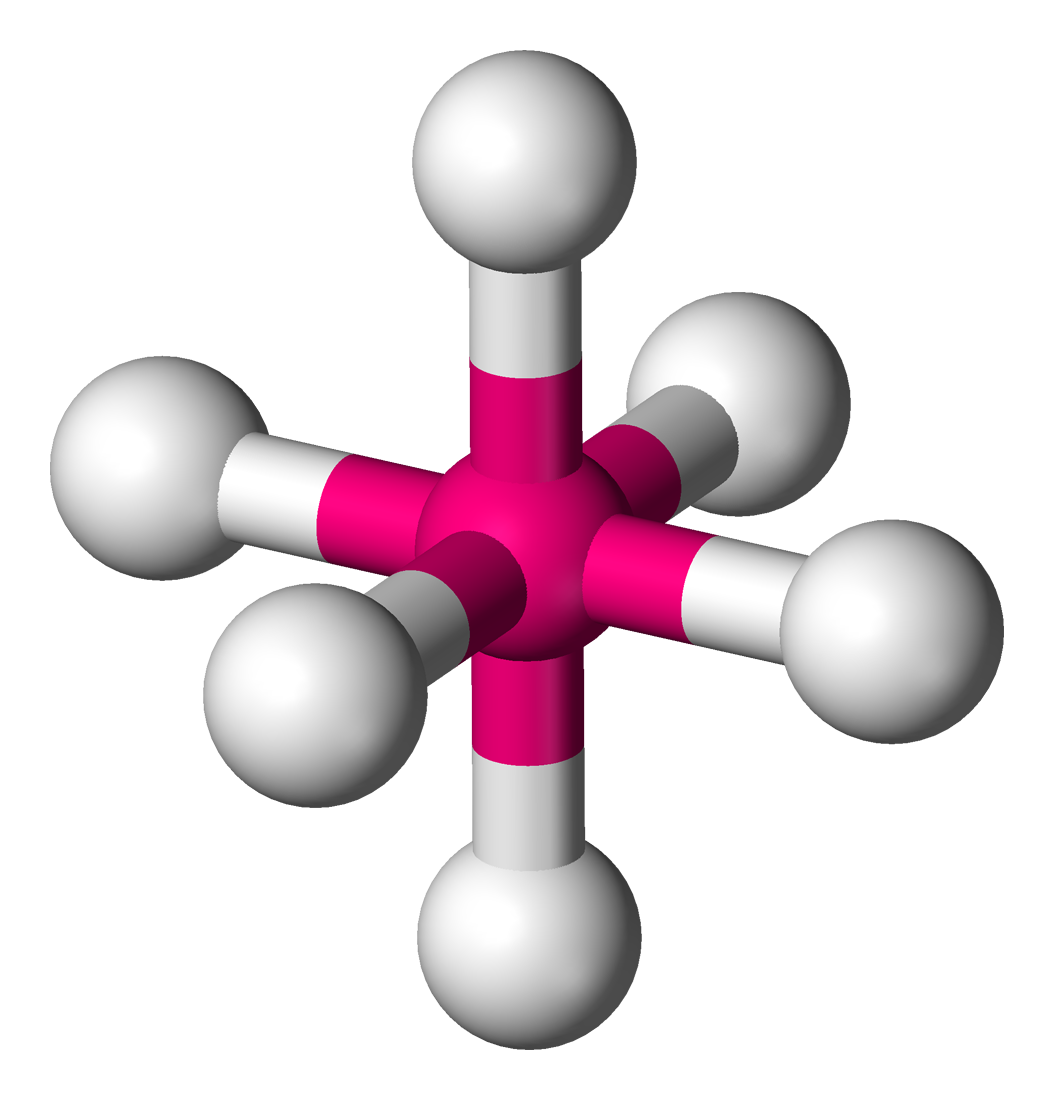
Square pyramidal
5 atoms on center
<90 degrees
1 lone pair
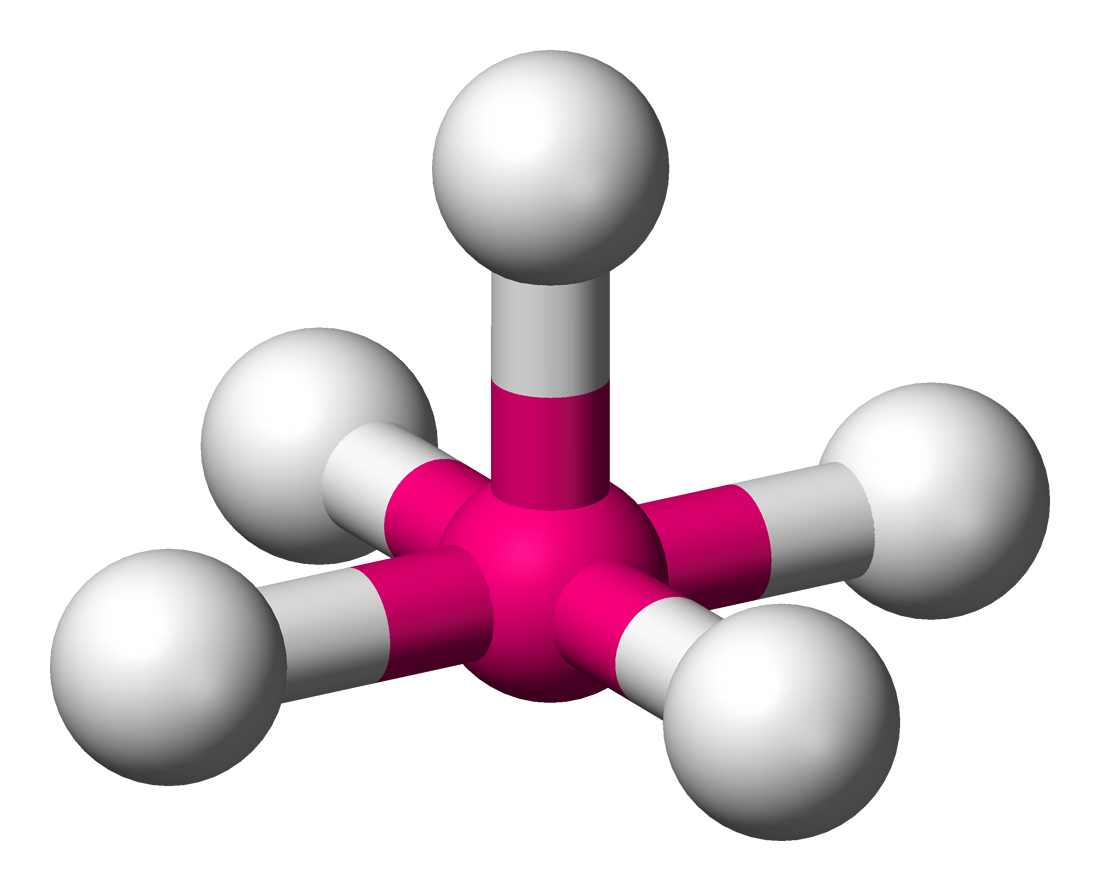
Square planar
4 atoms on center
90 degrees
2 lone pairs