Science exam semester one year 10
1/97
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
98 Terms
Balanced forces
Forces that are equal in size and opposite in direction
They cancel each other out
No change in motion or shape
Object may be still or moving at a constant speed
Example: A book resting on a table (gravity pulls down, table pushes up)
Unbalanced Forces
Forces that are not equal
Cause a change in motion (speed up, slow down, change direction)
Example: Two dogs pull a toy — if one dog pulls harder, the toy moves
Representing the forces on an object
Forces are shown with arrows
Arrow length = size of force
Arrow direction = direction of force
If the object is not moving or not accelerating, forces are balanced
Applied Force
A push or pull by a person, animal, or machine
Example: Holding a soccer ball — your hand applies a force upward
Balances gravity, so the ball doesn’t fall
Free Body Diagram
Object is shown as a square or box
Arrows show forces acting on it
Helps find the net force
Balanced Forces in Free Body Diagram
Equal length arrows in opposite directions
Forces cancel → net force = 0
No movement or constant speed
Support Force
The upward force from a surface
Balances gravity when something rests on a surface
If object doesn’t sink → support force
Net force
The sum of the forces acting on a single object
If the forces are balanced, the net force is zero → no change in motion.
If the forces are unbalanced, the net force is not zero → the object will accelerate (speed up, slow down, or change direction).
add any forces acting in the same direction and subtract any forces acting in opposite directions.
units of net force are newtons (N).
10 N force right, 6 N force left → Net force = 4 N to the right
5 N up, 5 N down → Net force = 0 N (balanced)
Newton’s first law of motion
Law of inertia- An object will remain at rest or move with constant velocity unless acted on by a net force
Properties of inertia
Inertia is not a force
It doesn't make something move or stop
It's a property of all objects with mass,
Moving vs stationary objects and inertia
Inertia is the natural tendency of an object to keep doing what it's already doing
An object at rest → stays at rest
A moving object → keeps moving at the same speed and direction
Inertia means an object resists changes to its motion
Velocity
Velocity = speed + direction
A change in velocity means a change in speed, direction, or both
Inertia keeps an object’s velocity the same unless a net force acts on it
Newton’s Second Law of Motion
Newton’s 2nd Law: F = ma
(Force = mass × acceleration)
Proportional Relationships (F=ma)
When the force acting on an object increases, the acceleration also increases.
→ This means acceleration is directly proportional to force.
(More force = more acceleration)
Force Units
1 Newton (N) = 1 kg × 1 m/s² (f=ma)
Standard units:
Force (F) → Newtons (N)
Mass (m) → Kilograms (kg)
Acceleration (a) → Metres per second² (m/s²)
Simulation uses:
millinewtons (mN) = 0.001 N
grams (g) = 0.001 kg
F = ma still works if you divide both mass and force by 1000
Why More Mass Needs More Force
More mass = more inertia
Inertia = resistance to change in motion
A greater force is needed to accelerate a larger mass
So: same force → smaller object accelerates more
Newton’s Third Law
Every action has an equal and opposite reaction.
Forces always come in pairs.
These pairs are called action-reaction forces.
Action-Reaction forces must be:
equal in size
opposite in direction
of the same type
acting on different objects
e.g. a horse pulls a cart (action), the cart pulls back on the horse (reaction). They don’t cancel out because they act on different things.
Labelling forces
Each label should have the following form:
Fforce type, x on y
For example, you hit a nail with a hammer. We could represent the force like this:
Fapplied, hammer on nail
Action reaction forces vs balanced forces
Equal and opposite forces only cancel out when they act on the same object.
This is when we say that they are balanced.
But, the pairs of forces described by the third law always act on different objects.
What is speed?
Speed describes how far something travels in a certain amount of time.
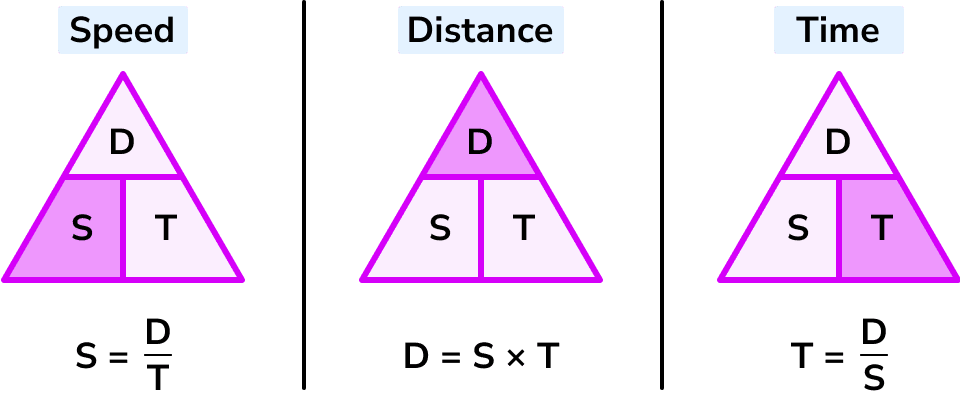
Speed Formula
speed= distance travelled/time taken
Converting between units of speed
To convert km/h → m/s, divide by 3.6
To convert m/s → km/h, multiply by 3.6
Units of speed- option 1
Speed- m/s
Distance- m
Time- s
Units of speed- option 2
Speed- km/h
Distance- km
Time- h
Instantaneous speed
An objects speed at any instant of it’s motion.
Average speed
The average of the instantaneous speeds over the whole distance travelled.
Average speed=Distance/Time
Transposing speed equation
What does a distance–time graph show?
How far an object has travelled over time.
x axis= time
y axis= distance
gradient= speed
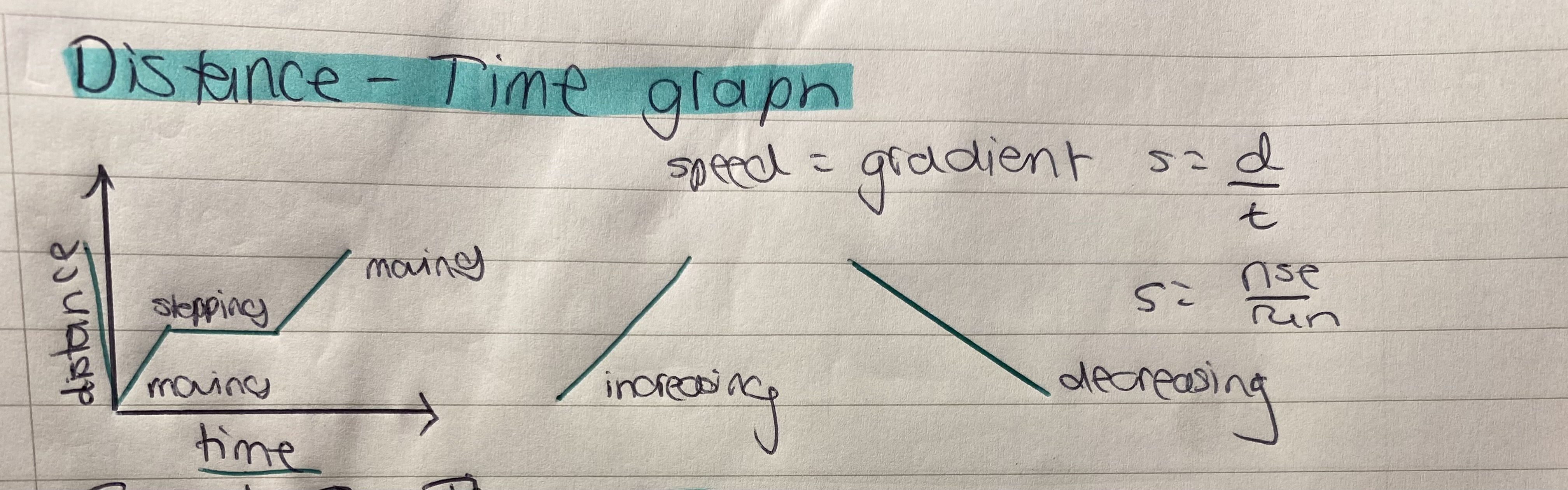
How to read a distance-time graph
Upward curve (steepening) = Speeding up
Curve flattening out = Slowing down
Straight line = Constant speed
Flat horizontal line = Stopped
How does the slope of a distance–time graph relate to speed?
The steeper the slope, the greater the speed.
The slope = speed.
How do we calculate average speed between two times?
Speed=gradient
s= rise(distance)/run(time)
speed= d2−d1/ t2-t1
Change in distance/ change in time

What does a speed–time graph show?
How fast an object is moving at each moment.
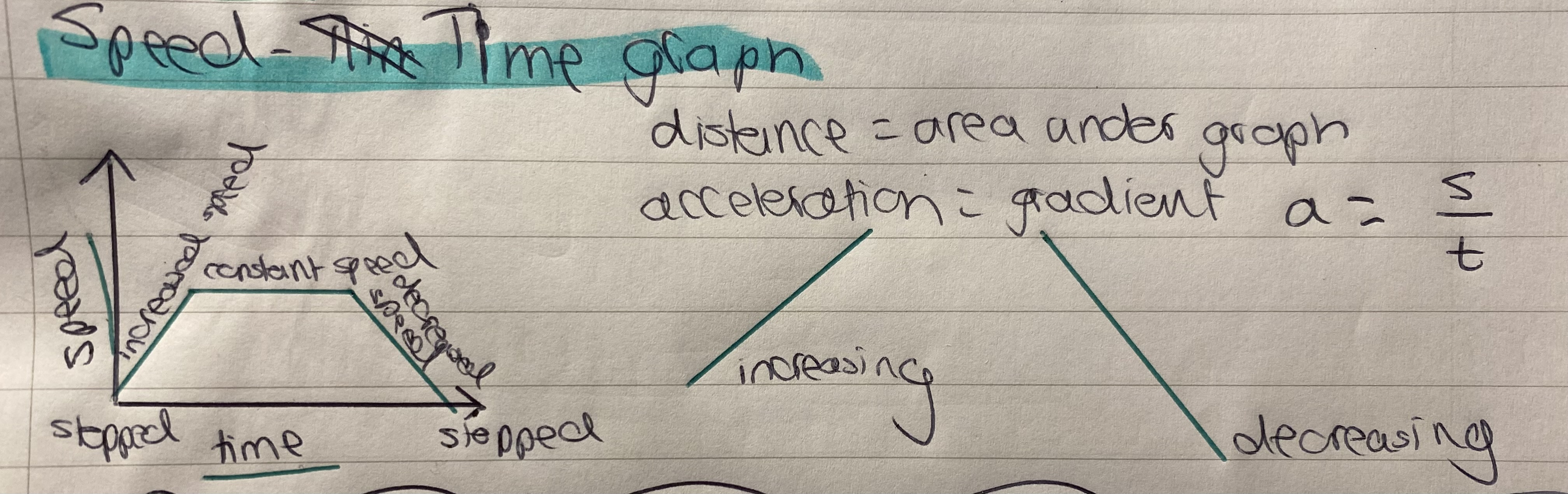
How to read a speed-time graph
Upward slope (line going up)
→ Acceleration (speed is increasing)Downward slope (line going down)
→ Deceleration (speed is decreasing)Horizontal line (flat)
→ Constant speedLine at zero (on the time axis)
→ Object is stopped / not moving
Distance
Total distance travelled during the motion
Displacement
Straight line distance from an object’s starting point
has magnitude(size) and direction.
e.g. it's 120 km by a slow and winding road between Snake Gully and Gusville. But the map shows Gusville is only 70 km east of Snake Gully.
So, the displacement from snake gully to Gusville is 70km east
Scaler quantity
Has magnitude(size) but not direction
Distance is a scalar quantity
Speed is a scalar quantity, because it is measured using distance and time, which are both scalar quantities.
Vector quantity
Has magnitude(size) and direction
Displacement is a vector quantity.
Velocity is a vector quantity
Velocity formula
Velocity= displacement/time
e.g. A map shows Gusville is only 70 km east of Snake Gully.
If it takes a driver 2 hours to get between the towns, then their average velocity is: 70/ 2
= 35km east
What's the difference between average speed and average velocity?
Speed = uses distance
Velocity = uses displacement
They’re often different!
Positive and negative velocity
Choose a direction as positive.
Move that way = positive velocity
Move opposite = negative velocity
( Right and up is often positive, left and down is often negative)
What is Acceleration?
Acceleration = any change in velocity
Velocity = speed + direction
It is a vector quantity
So, acceleration happens when:
🚀 Speed increases
🐢 Speed decreases
🔄 Direction changes
Acceleration Formula
Acceleration= change in velocity/time taken
a= v2-v1/t2-t1

Standard units of acceleration
a= m/s/s, m/s2 , or ms-2
v= m/s or ms-1
t=s
Positive vs Negative Acceleration
Positive acceleration = speeding up
Negative acceleration = slowing down (also called deceleration)
⚠ But if you're moving in a negative direction, negative acceleration can mean speeding up in that direction!
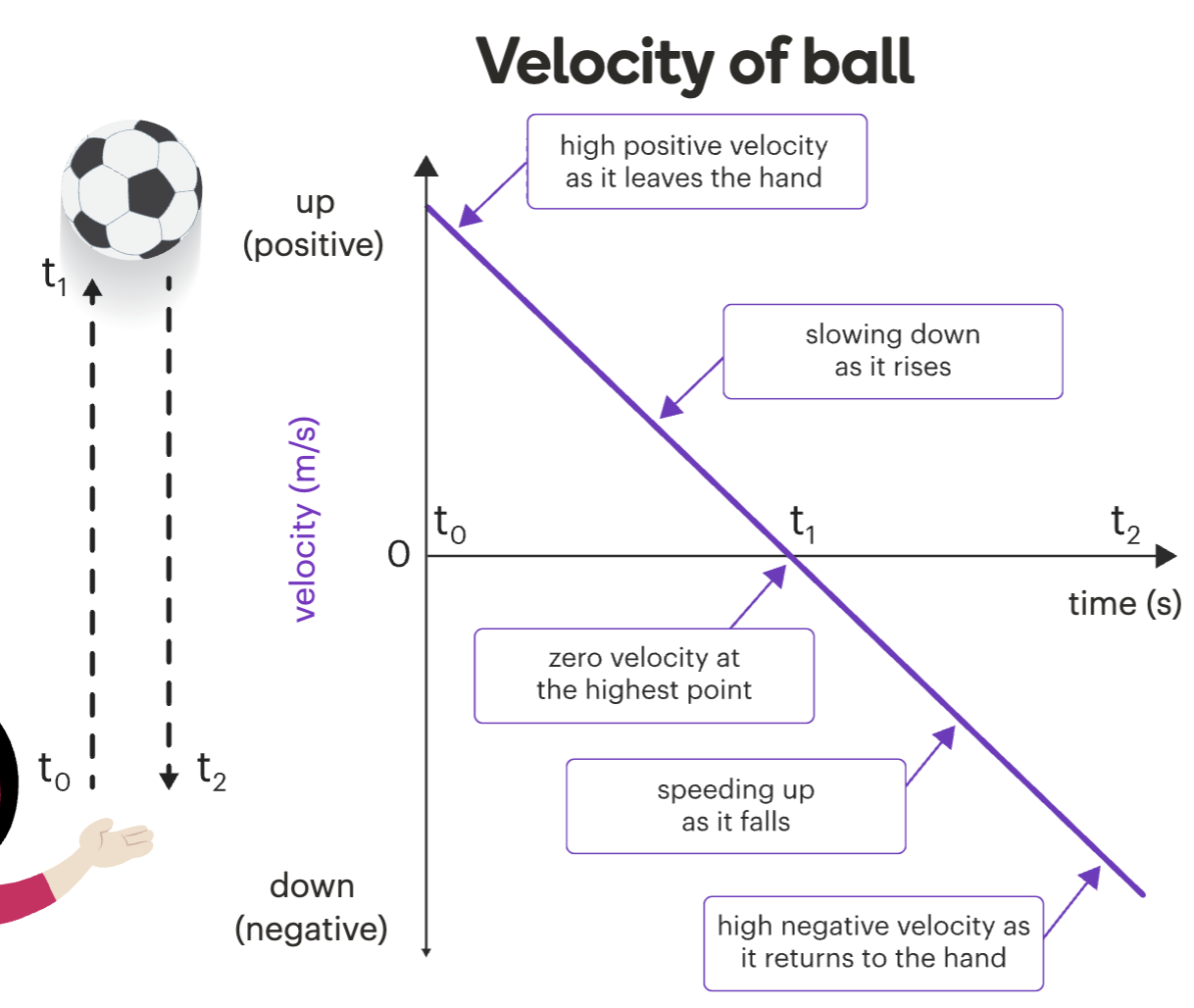
Velocity-Time graphs: slope
The slope (tilt) tells you the acceleration:
Upward slope = Positive acceleration (speeding up in + direction)
Downward slope = Negative acceleration (slowing down or speeding up in – direction)
Flat line = Constant velocity- same speed and direction (zero acceleration)
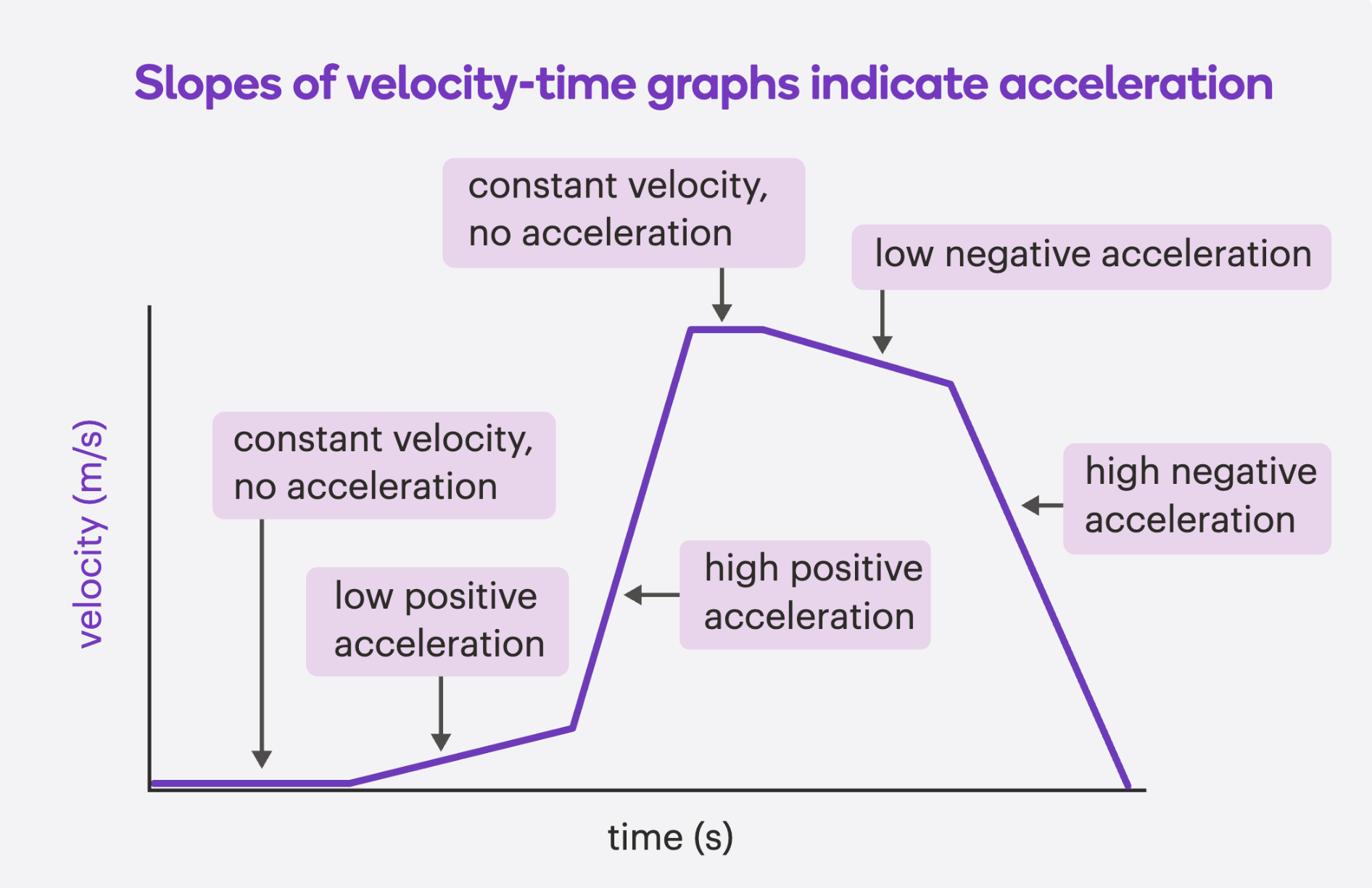
Velocity-Time graphs- other important info
On a velocity-time graph:
Area under the line = distance travelled
Slope of the line = acceleration
Steeper = faster acceleration or deceleration
Flat = no acceleration
What is acceleration due to gravity?
The same force that acts on all objects on Earth.
Acceleration due to gravity (g) has a value of about 10 m/s/s down.
It always acts downwards towards the centre of the Earth, no matter the direction the object is going
Why would an object have a negative acceleration even when going up?
Because gravity pulls it down the whole time,
Slows it down going up
Speeds it up coming down
What does a straight, sloping line mean on a velocity-time graph?
Constant acceleration
Negative slope = acceleration is downward
Line goes through 0 when an object changes direction
Physical change
A change in matter that does not form new substances.
Examples of physical changes
Change in
Position
Shape
Size
State
Chemical change/reaction
A change in matter that forms one or more new substances.
Examples of chemical changes
release of light or sound
Formation of a new gas
Change in colour
Disappearance of a solid
Formation of a new solid
Change in temperature
Atom
The smallest particle of an element
Molecule
A group of atoms bonded together
Fixed formula, e.g. O2, H2O
Chemical bond
An attractive force that holds two atoms together
Mixture
A combination of substances that can be physically seperated.
Element
a pure substance made up of only one type of atom.
Compound
Made up of two or more different types of atoms bonded together, such as carbon dioxide (CO2) and sodium chloride (NaCl).
Chemical equation
Reactants—>Products
e.g. sodium + oxygen → sodium oxide
Reactants
The substances that react with each other
Products
The new substances formed by a reaction
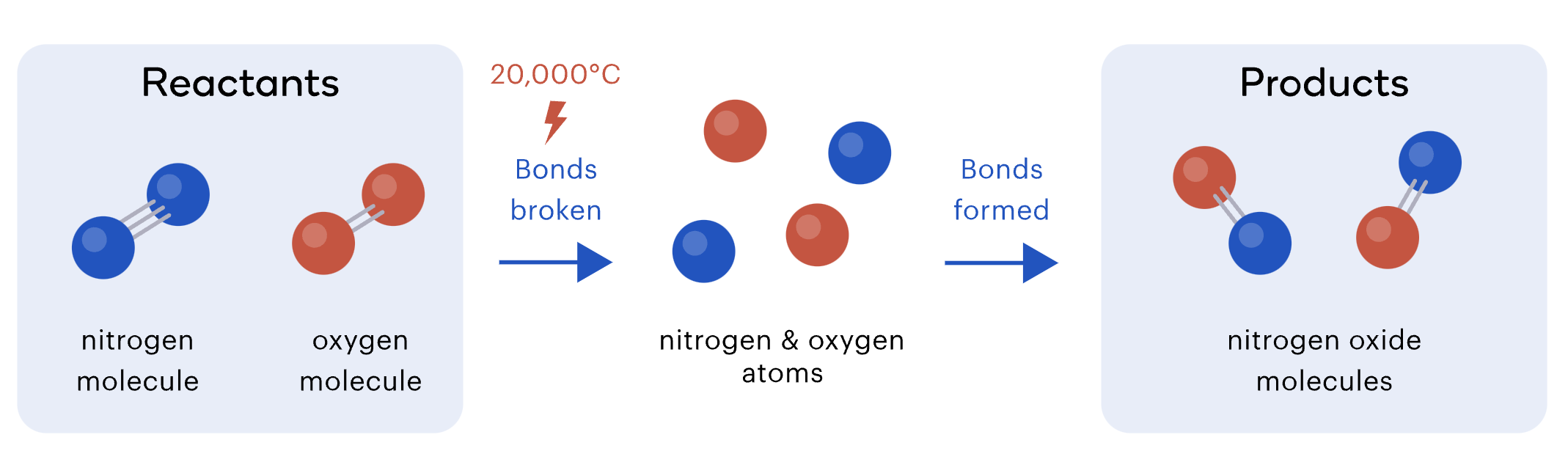
The re-arrangement of atoms
During a chemical reaction, some of the chemical bonds between atoms are broken and new bonds are formed.
This re-arrangement of atoms is what produces a new substance.
The same elements are present after a reaction – they're just arranged in a new way.
Metal atom
1, 2, or 3 electrons in outer shell
Non-metal atom
5, 6 or 7 electrons in outer shell
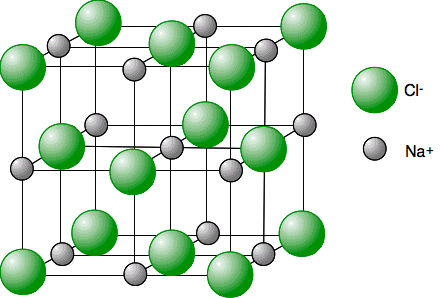
Lattice
Continuous arrangements of bonded atoms in regular patterns.
Ratio of elements, e.g. NaCl, Au
Why do elements bond together?
Atoms form chemical bonds to obtain full valence shells.
By bonding together in chemical reactions, atoms can reach a more stable state.
Ions
Charged particles, which are formed when atoms either lose or gain electrons
Cations
Positively charged ions formed by the loss of electrons
Anions
Negatively charged ions formed by the gain of electrons
Ionic bonds
The transfer of electrons from one atom to another results in two ions with opposite charges. The attraction between these opposite charges is what makes an ionic bond.
Occurs between metals and non metals
Metal cations
Non-metal anions
Predicting ionic compounds
E.g: Aluminium + Chloride
Aluminium- Metal, charge of 3+
Chloride- Non-metal, charge of 1-
This means you need three negative charges to balance out the positive charge of three.
(Al³⁺)+ (Cl⁻)+ (Cl⁻)+ (Cl⁻)
= AlCl₃
metals are always written before non-metals
Metallic bonding
When metals bond with metals, they donate their valence electrons into a common pool.
This results in metal cations floating on a sea of delocalised electrons
The positively charged metal ions (cations) are held together by the attraction to this sea of delocalized electrons.
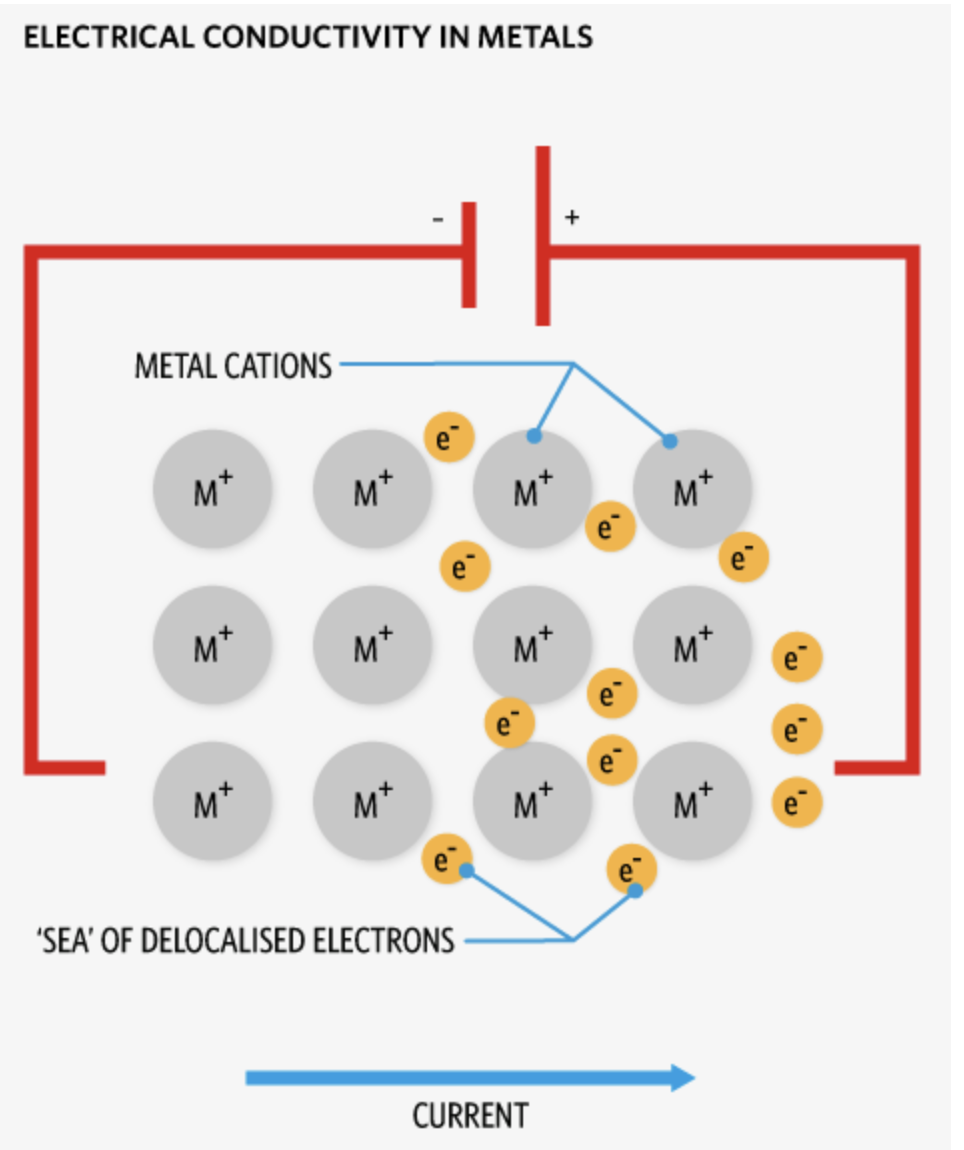
Properties of metals explained by metallic bonding- Electrical Conductivity
Delocalised electrons can move freely through the metal- a flow of electrons is an electric current
Properties of metals explained by metallic bonding- Heat Conductivity
The delocalised electrons carry thermal energy (heat) through the metal quickly and easily.
Properties of metals explained by metallic bonding- Shine
Delocalised electrons move quickly so that light can reflect off all surfaces of the metal.
Covalent bonding
When non-metals bond with non-metals, they share a pair of electrons between atoms.
Each atom contributes one or more electrons to form a shared pair.
One pair represents two total electrons
This sharing allows both atoms to achieve a stable electron configuration (a full outer shell).
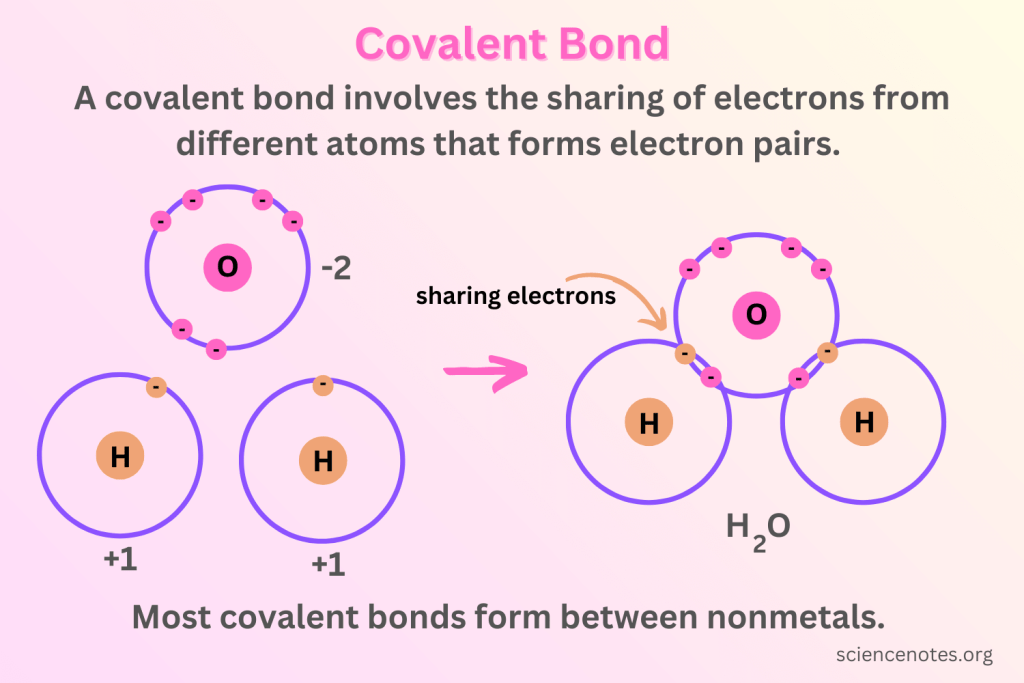
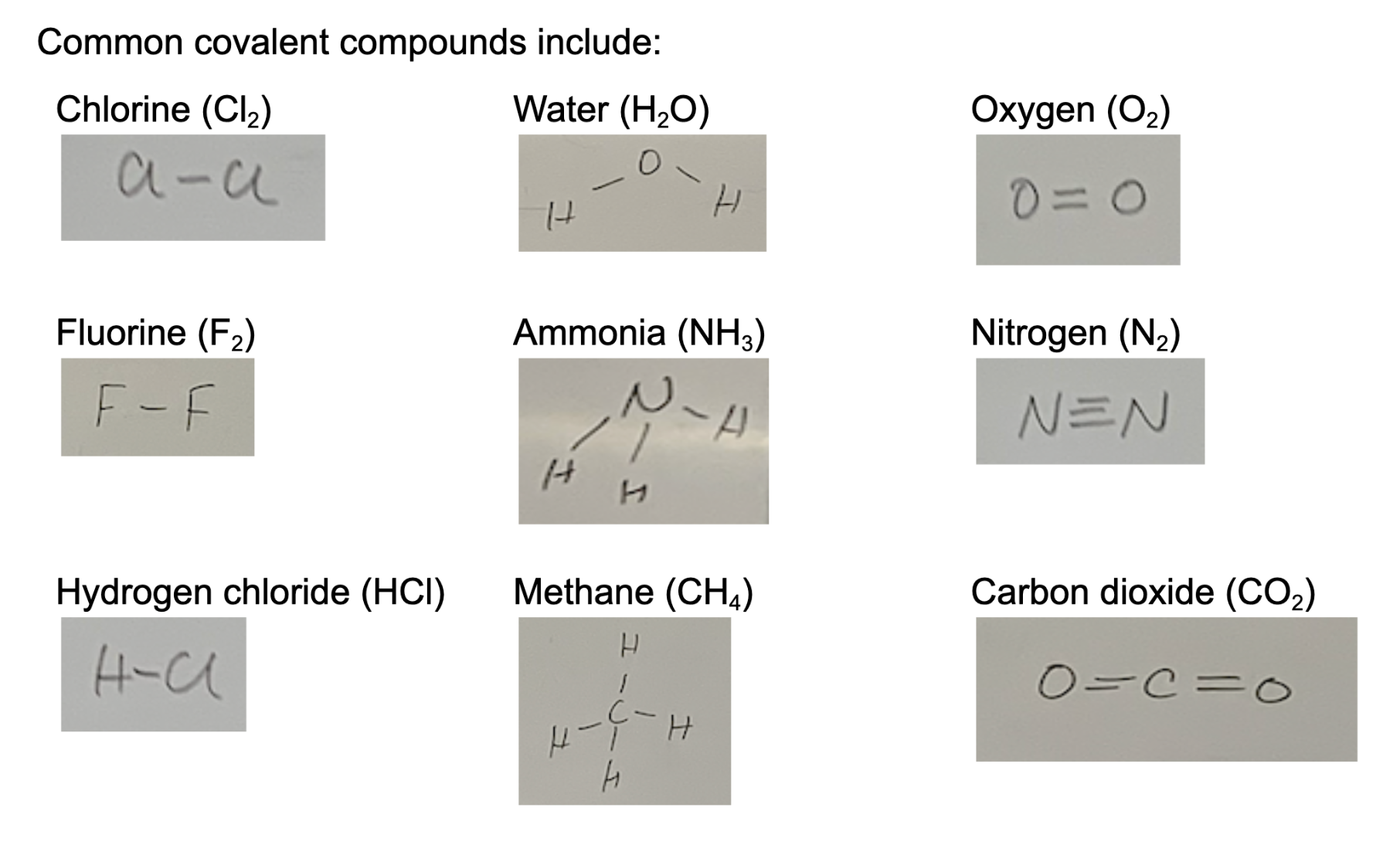
How to represent covalent bonding
F-F, H-H, H-O-H
The number of lines between the element symbols represent the number of bonds
Bonds of common elements
H, F, Cl- single bond ( two electrons are shared)
O- double bond(four electrons are shared)
N- triple bond( six electrons are shared)
C- quadruple bond( 8 electrons are shared)
Law of Conservation of Matter
In a chemical reaction, atoms are not created or destroyed.
Total number of atoms of each element remains the same before and after the reaction.
The bonds of the atoms are just rearranged in different ways, forming new substances.
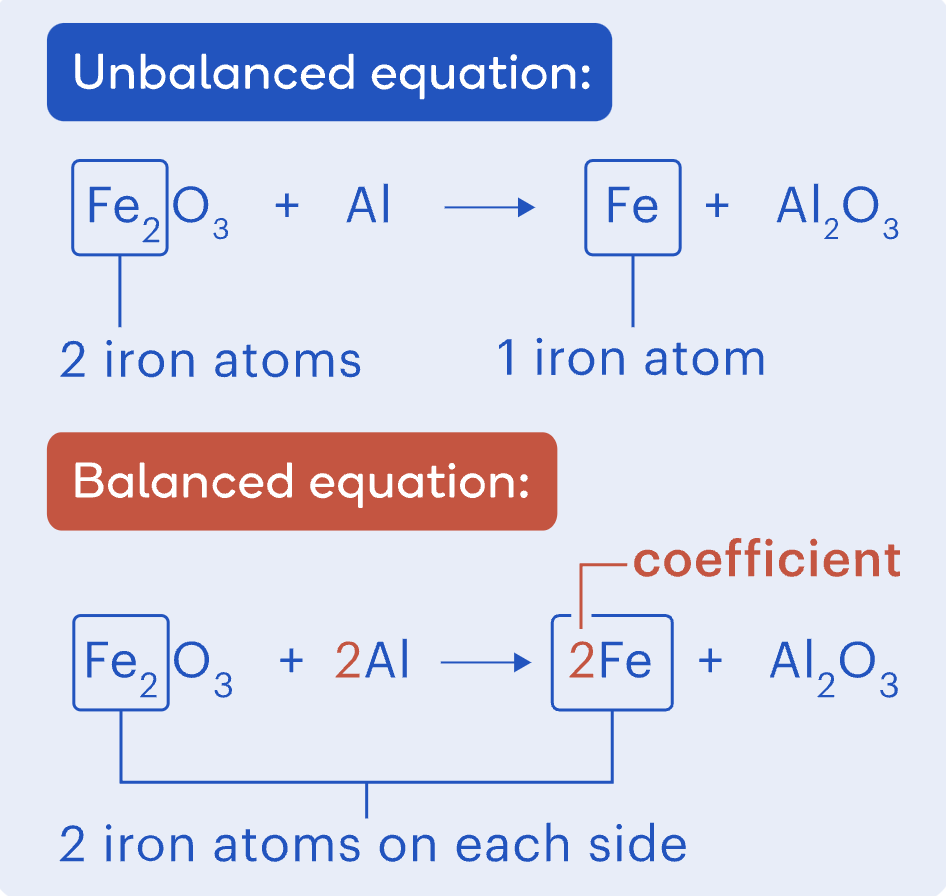
Unbalanced Equations
An equation is unbalanced if the number of atoms of each element is not the same on both sides.
Example (Unbalanced Thermite Reaction):
Fe₂O₃ + Al → Fe + Al₂O₃
The number of Fe and Al atoms do not match.
Balancing equations
An equation is balanced when the number of each type of atom is the same on both sides.
To balance an equation:
Adjust the numbers in front of chemical formulas (coefficients).
Example (Balanced Thermite Reaction):
Fe₂O₃ + 2Al → 2Fe + Al₂O₃
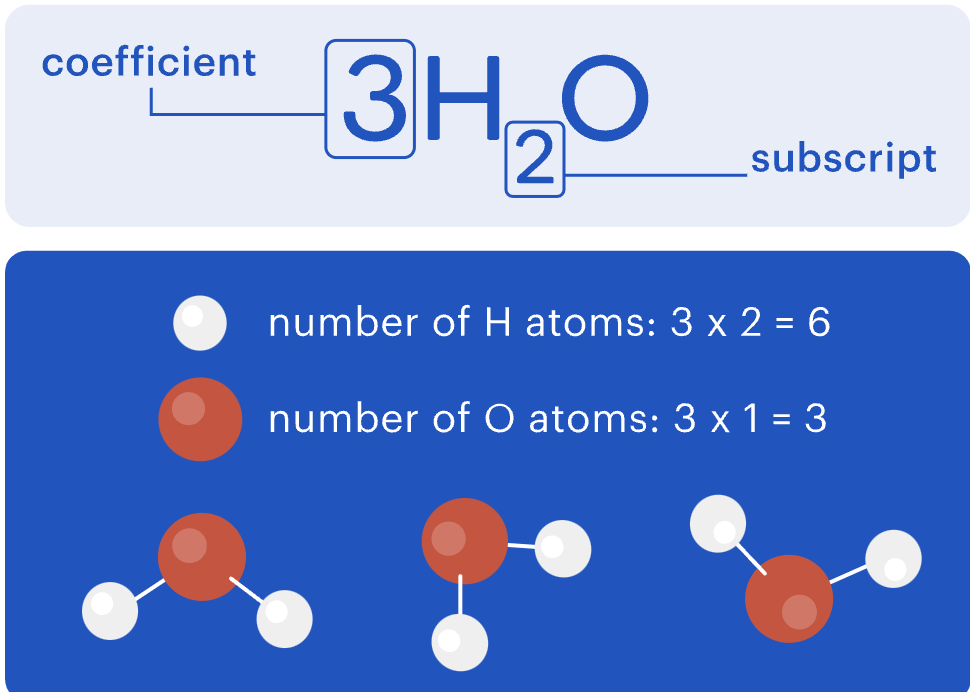
Subscripts and coefficients in chemical equations
When balancing an equation, you can’t change the subscript numbers, as this shows how the molecule is naturally formed.
You can only adjust the coefficients, which change the number of elements or compounds in a chemical equation.
To find the amount of each type of atom, multiply the coefficient by the subscript
How to balance equations
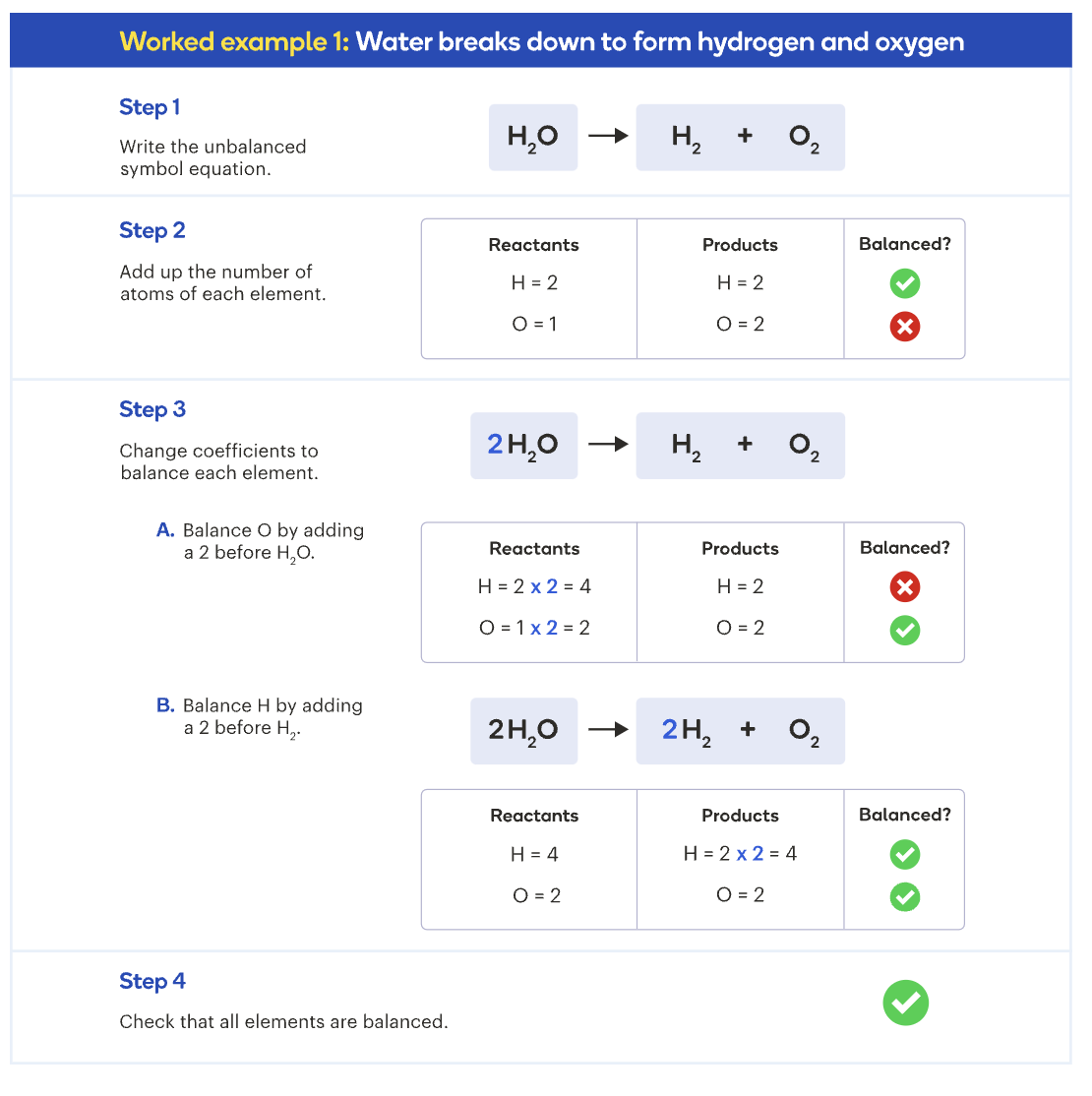
Composition reactions
Two or more reactants combine to form a single product.
General form: A + B → AB
Examples: N2+3H2→2NH3, 2Mg+O2→2MgO
multiple reactants, single product
Decomposition reactions
One reactant breaking down into multiple products
General form: AB→ A + B
Examples: 2H2O→2H2+O2, CaCo3→ CaO+CO2
Single reactant, multiple products
Displacement reaction
One element replaces another in a compound
General form: AB+C→BC+A
Examples Zn+CuSO4→ZnSO4+Cu, Cu + AgSO4→2Ag+CuSO4
One metal dissolving, another coming out of solution
Often two metals involved
Neutralisation reaction
Acids and bases reacting together
General form: Acid + base→water + salt
Examples: HCl+ NaOH→H2O+NaCl, H2SO4+Mg(OH)2→2H2O + MgSO4
Acid, base
Precipitation reactions
Two solutions are mixed and a solid forms
General form: AB+CD→ AD+CB (one product is solid)
Examples: Pb(NO3)+2KI→ PbI2+2KNO3
Solid forms from mixing solutions
Three main types of chemical bonding
metal + metal → metallic bonding
metal + non-metal → ionic bonding
non-metal + non-metal → covalent bonding
Collision theory
The idea that the re-arrangement of atoms requires collisions between the reactant particles.
It's only when the particles are in contact that new bonds can form to make the products.
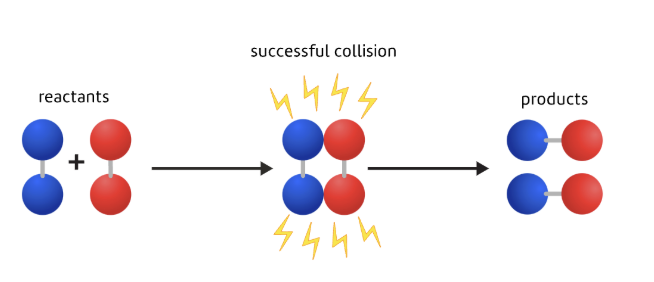
What a successful collision involves
Particles colliding with both
the right orientation, and
enough energy, or speed, to break their bonds
If both conditions aren't met then the particles will just bounce off each other without reacting.
Rate of a reaction
How quickly reactants are converted into products.
The higher the frequency of successful collisions, the higher the reaction rate.
What you need to increase the rate of a chemical reaction:
surface area
temperature
concentration
These all increase the chance of a successful collision
Surface area
Surface area is the total outside area of a three dimensional solid.
As a solid is broken into smaller prices, the surface area increases.
When the surface area is increased, the chance of reactant particles colliding increases, so there are more reactions and the reaction is faster.
Temperature
When the temperature is increased, the average kinetic (movement) energy of the particles is increased, so particles move faster.
More collisions occur, and more particles have enough energy to react. This means there are more reactions and the reaction is faster.
Concentration
Concentration is how many particles there are per unit volume.
When the concentration is increased, the chance of reactant particles colliding increases, so there are more reactions and the reaction is faster.