28 - Heme Synthesis and Erythrocytes
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
What are some characteristics of RBCs.
RBCs (as well as leukocytes, white blood cells, and platelets make up the blood.
Consist mostly of hemoglobin (contains red pigment, making RBCs red.
Do not contain organelles or nuclei, maximizing available volume for hemoglobin.
Have disc-like shape with central depression that is half the thickness of the outer area, giving a biconcave cross section. Gives the cell an excess of membrane and flexibility for gas exchange and fitting through capillaries
What are some characteristics of the lipid bilayer of red blood cells? What proteins maintain RBC structure?
The lipid bilayer contains integral membrane proteins and peripheral membrane proteins, which come together in a lattice-like network called the cytoskeleton
Spectrin (most abundant protein in cytoskeletal lattice followed by actin), which forms heterodimers (a- and B-spectrin)
Dimers join at junctional complex (formed by actin) band 4.1, and actin-binding proteins. The complexes are tethered to the cell membrane by glycophorin (membrane protein)
Ankyrin (band 3, 4.1, and 4.2) anchor lattice to cell membrane
What are common symptoms of porphyria?
Sensitivity to sunflight, anemia (paleness), accumulation of porphyrins (flourescent teeth), gingival recessive (fang appearance), disfigured facial features, fingers, toes, corneal scarring due to sunlight exposure
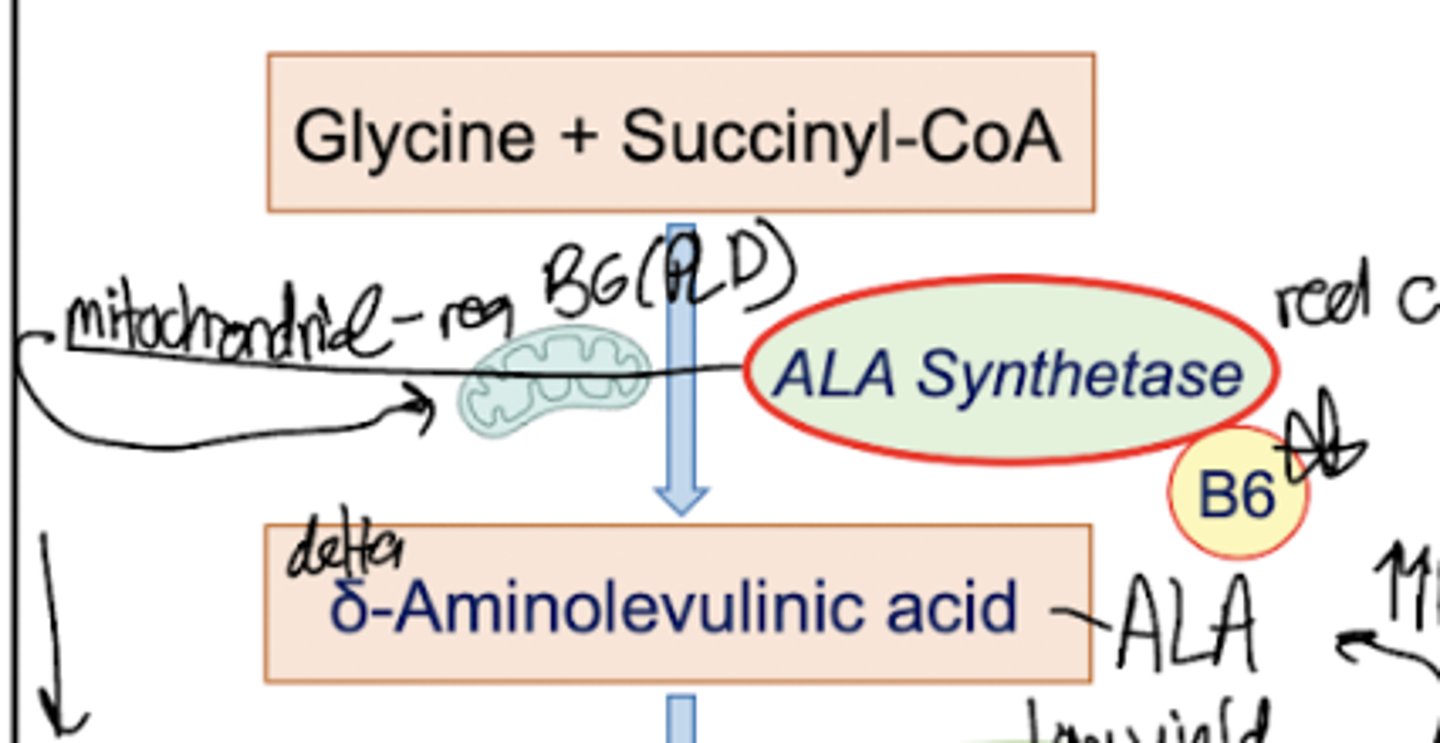
What is step one of heme synthesis?
Glycine is combined with Succinyl CoA, with required PLP to form delta-Aminolevulinic Acid. The enzyme involved is ALA synthetase and this reaction takes place in the mitochondria.
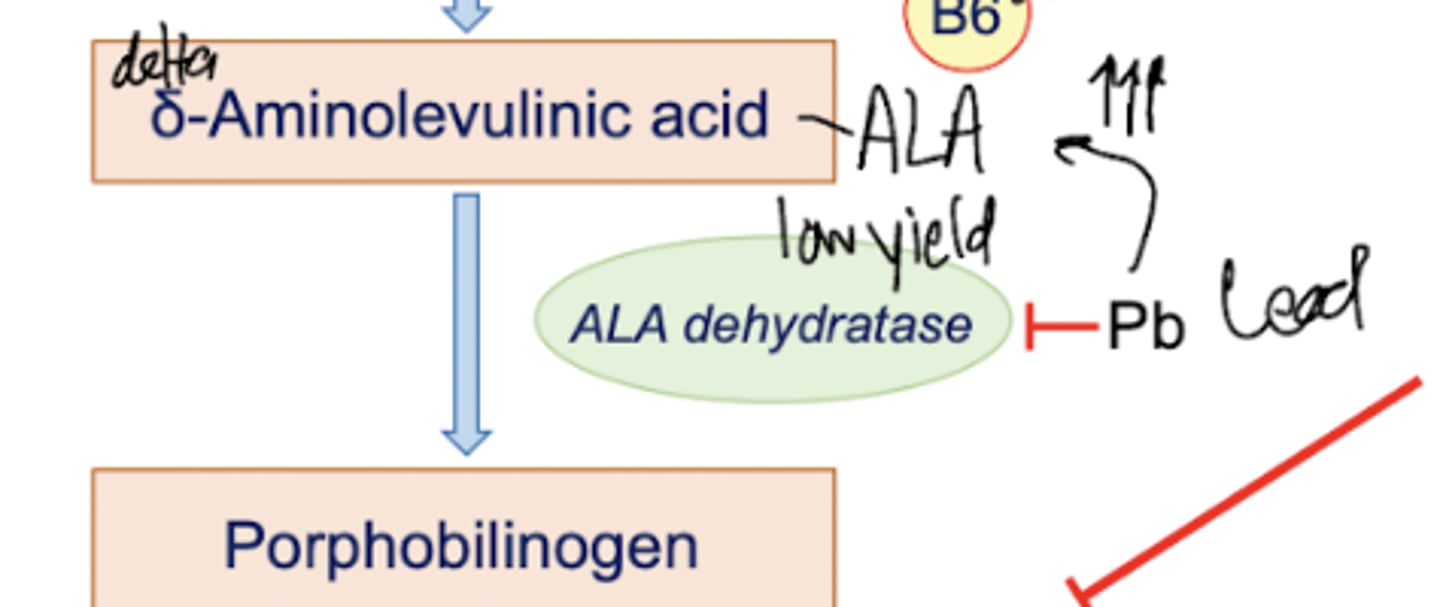
What is step two of heme synthesis?
ALA is converted into porphobilinogen by ALA dehydratase. This cytosolic enzyme is inhibited by Pb (lead), but no one really gives a damn because this enzyme is low yield.
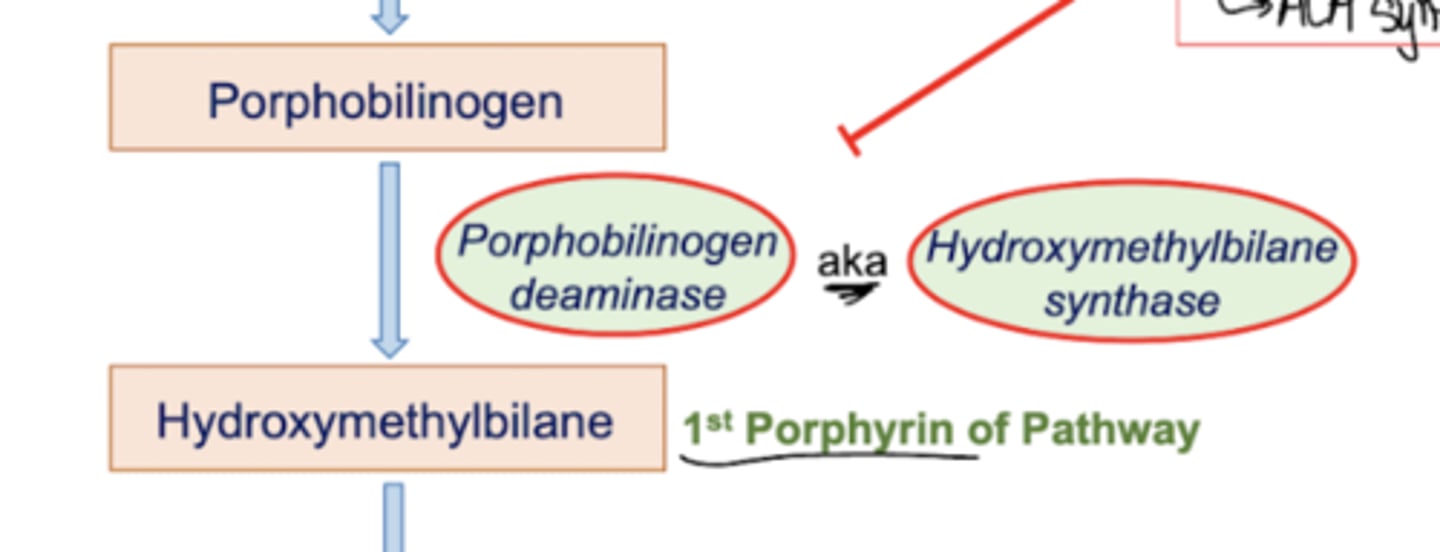
What is step three of heme synthesis? What are some involved pathology?
Porphobilinogen is converted into hydroxymethylbilane (the first porphyrin of the pathway) by an enzyme with multiple identities. It can either be referred to as porphobilinogen deaminase, OR hydroxymethylbilane synthase.
Now, this enzyme is involved in some f** s**. Acute Intermittent Porphoryia is a disease involving the deficiency of PB deaminase/HMB Synthase. It is an autosomal dominant, late onset disease. It involves episodic anxiety, confusion, paranoia, and acute abdominal pain. There is NO PHOTOSENSITIVITY in this disease. There may be port-wine colored urine in some patients. Patients with this disease CANNOT have barbiturates (they increased ALA synthetase activity, activating the pathway to no avail)
What is step four of heme synthesis?
Hydroxymethylbilane is converted into Uroporphyrinogen-III by Uroporphyrinogen-III synthase. This enzyme is not an emphasized step, and thus does not get any cool points.
What is step five of heme synthesis?
Uroporphyrinogen-III is converted into Coproporphyrinogen-III by Uroporphyrinogen decarboxylase.
This enzyme is ALSO involved in some shtuff. Porphyria Cutanea Tarda is a disease that is the most common porphyria. It is autosomal dominant, with a late onset. Some symptoms include inflammation, blistering, shearing of skin area exposed to light, hyperpigmentation, PHOTOSENSITIVITY, and red-brown to deep-red urine. These symptoms are exacerbated by alcohol. These patterns also CANNOT get barbiturates (activate pathway)
What is step six of heme synthesis?
Coproporphyrinogen-III is converted into Protoporphyrin-9 by two enzymatic reactions: coproporphyrinogen oxidase and protoporphyrin oxidase.
What is step seven of heme synthesis?
Protoporphyrin-9 is converted into Heme by the addition of iron through the enzyme Ferrochelatase. This enzyme is MITOCHONDRIAL…
So, since both step one and step seven are in the mitochondria, they are able to directly interact (they’re “in the same room”). Thus, heme inhibits ALA synthetase.
Also, if there’s no iron hanging around to be tossed into P9, Zinc will be tossed in instead.
What do the suffixes -in and -ogen mean?
The suffix -in means a thing is colored
The suffin -ogen means a thing is colorless
What is plumbism?
Lead poisoning, as it inhibits Ferrochelatase.
Also may not work in SEVERE iron deficiency.
So, Zn will go in, leading to a colorless red blood cell that is fluorescent under a fluor microscope.
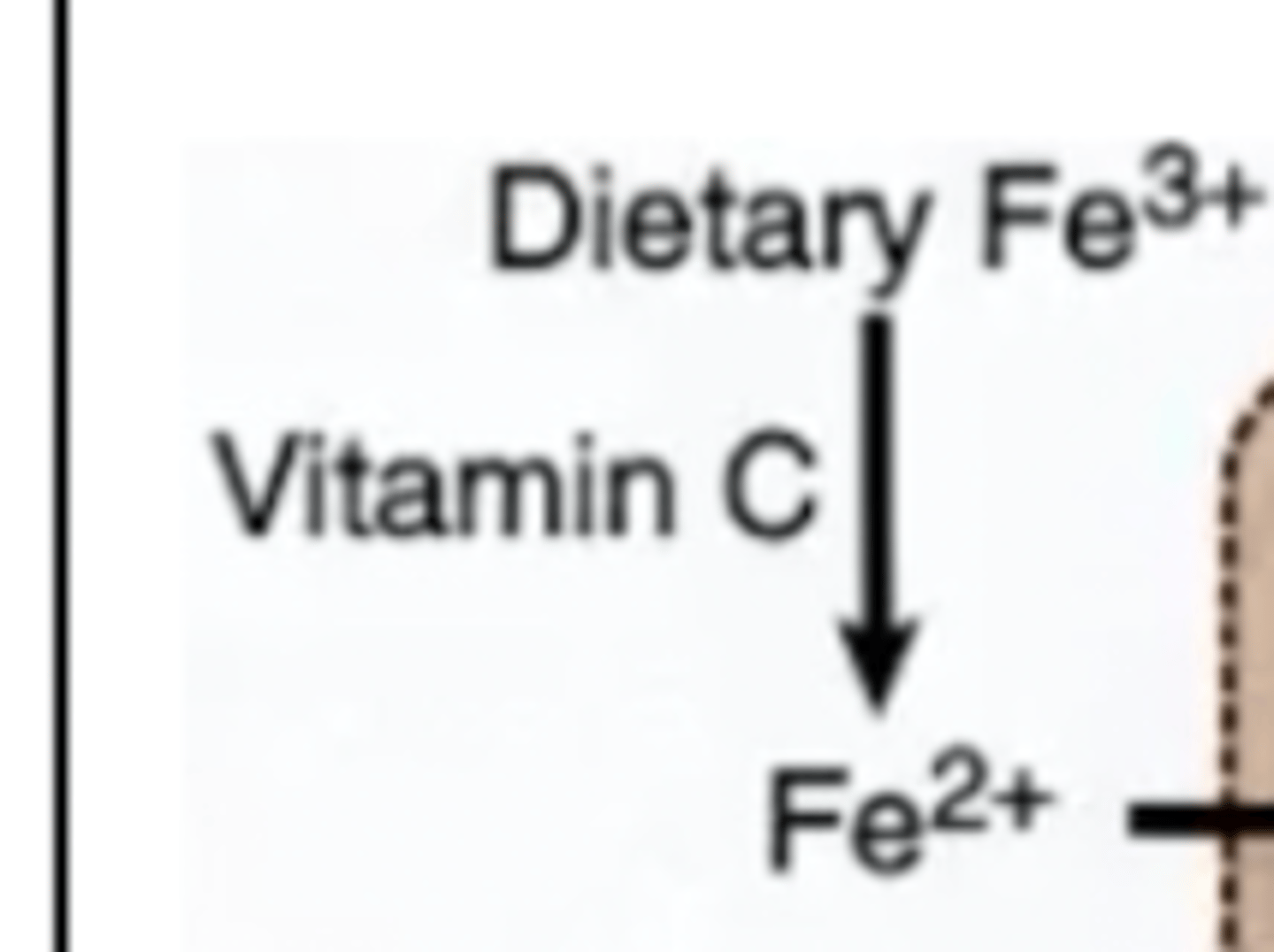
Describe what happens when dietary Fe is eaten? What state is it in?
This interaction forms from the interaction with vitamin C. About 10% of dietary iron is actually absorbed (about 1 mg per day - iron is never gotten rid of)
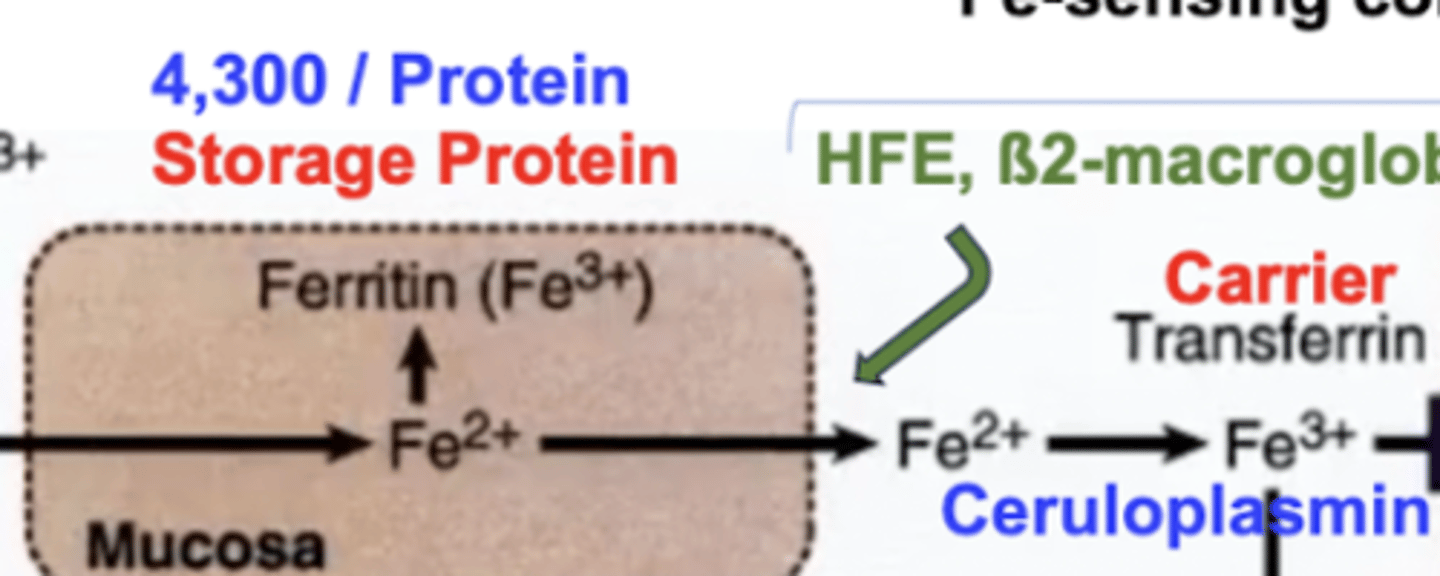
Describe what happens when iron enters the intestinal mucosa?
Iron has the freedom of tagging along with Ferritin (the iron binding protein) as ferric iron in order for storage, or it can be transported to where it is required in the blood by Transferrin. This requires some things though (see below):
Ferritin can store up to 4,300 iron molecules per protein, and is notably known as the iron storage protein
Hemochromatosis is a disease caused by iron overload (ferritin carries greater than its carrying capacity)
Hemosiderin deposits in the liver (leads to cirrhosis), pancreas (leads to diabetes), joints (arthritis), and skin (dermatitis).
Iron-sensing complex consists of HFE, B2-macroglobulin, and other funny proteins. This complex, with hepcidin as its control, allows iron to be released into the blood for transport by transferrin. Ceruloplasmin oxidizes iron (ferrous) into iron (ferric) to bind to transferrin.
Ceruloplasmin AKA ferroxidase requires Cu (copper).
Iron MUST be bound to something in order to traverse the body, it CANNOT be by itself, or else it will form complexes with things it’s not supposed to.
Describe what happens when iron enters most tissues.
Iron enters most tissues through the binding of transferrin to its receptors, releasing iron into tissues. This iron can be stored with ferritin, OR can go play with many enzymes and cytochromes
Describe what happens when iron into bones
Iron can be transported (same by transferrin) into the bone for erythropoiesis (RBC formation)
Describe what happens to iron after RBC turnover?
RBCs get demolished and Reticuloendothelial System cells carry iron into the bone for erythropoiesis
Describe the meaning of different levels of TIBC?
TIBC (total iron binding capacity).
Transferrin should be about ⅓ saturated.
High TIBC means iron deficiency (a lot more could be bound).
Low TIBC means iron overload (too much).
Hemochromatosis treatment is chelation therapy (remove iron).
Phlebotomy (removal of blood) or Deferoxamine (binds Fe for urinary secretion).
What are some characteristics of hemoglobin?
tetrameric protein that carries oxygen in the blood.
Heme in Hb is a tetrapyrrole ring system (protoporphyrin 9 with iron).
Each heme surrounded by globular polypeptide globin chain.
A-globin and B-globin
Heme binds oxygen - His-93 (prox) complexed to iron and His-64 (distal) hydrogen bond to oxygen.
Oxygen binding pulls Fe and His toward the ring plane
R state activate and T state inactive.
How does 2,3 BPG affect Hb affinity?
2,3 BPG is an allosteric effect that lowers the affinity (right shift) for oxygen.
It is negatively charged and binds to the six charged groups of dHb.
How does the Bohr effect affect Hb affinity?
lowering pH decreases affinity for oxygen (right shift)
How does carbamate affect Hb affinity?
CO2 can be transported by dissolved bicarbonate and carbamate adducts to hemoglobin.
Causes right shift, less affinity.
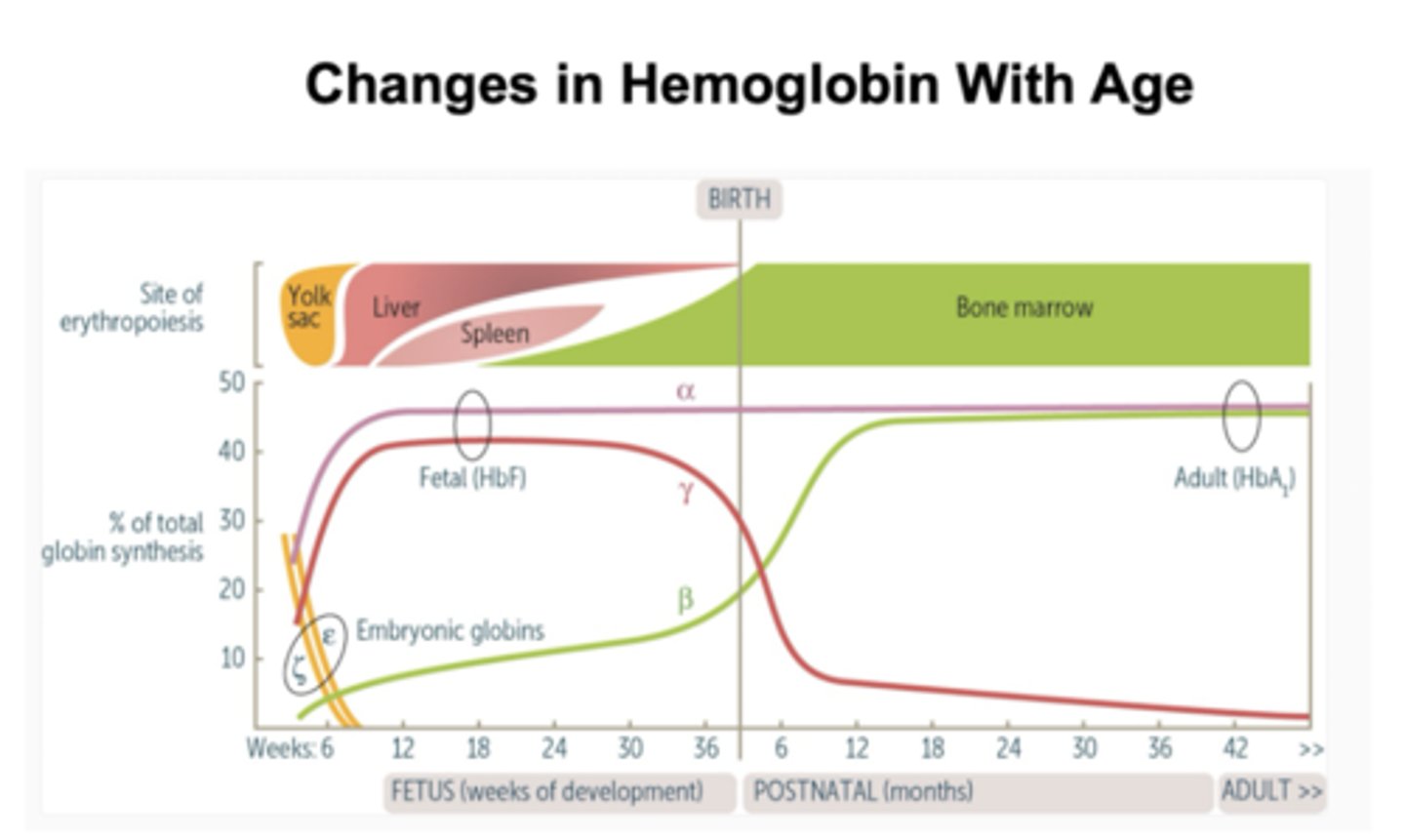
How does hemoglobin change with age?