Chemistry Unit 3 Study Guide
Ionics
Ions
Metals ALWAYS form cations (+) because they lose electrons
Cation —> t —> +
Nonmetals form anions (—) because they gain electrons
Anion —> n —> negative —> —
Every element wants a noble gas configuration
Compounds are ALWAYS neutral
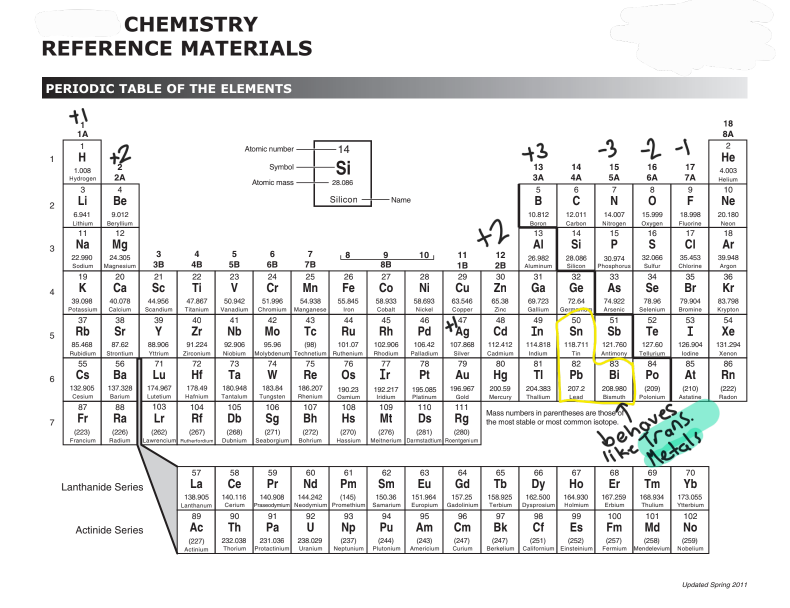
Transition Metals
Most can have multiple charges because they can have multiple valence electrons. Exceptions are shown in table
Use Roman Numerals to indicate charge
Fe (II) or Mn(VI)
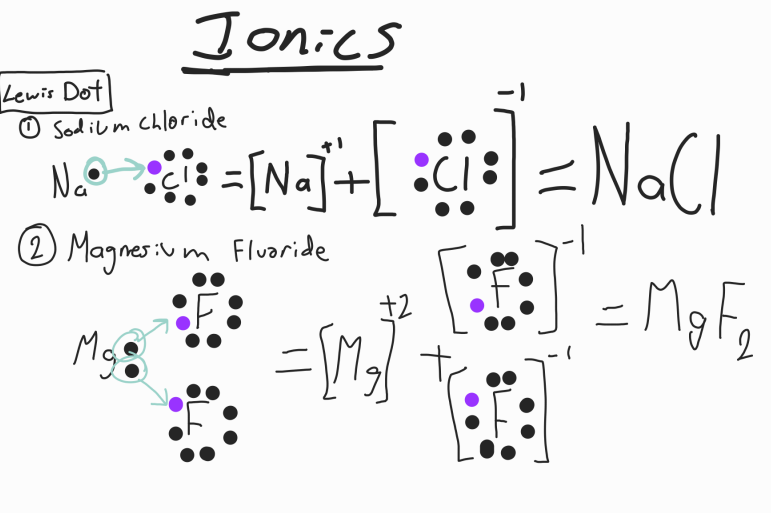
Lewis Dot Structure
Shows the transfer of electrons between Metals and Nonmetals
Formula Writing
If you know name, use the steps to find the formula. If you know formula, work backwards.
Write element symbols
Write charges
Swap, drop, and make positive
Factor
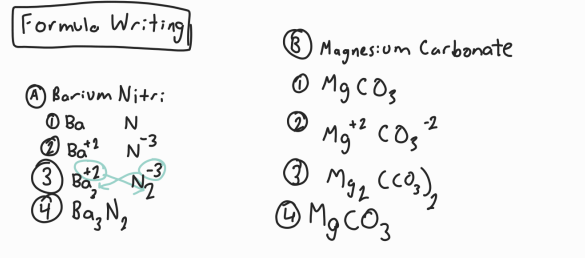
Polyatomics
These are compounds that behave like elements. Use “( )“ to indicate the number of polyatomics
Naming Ionics
Name the cations first (positive charge)
Name the anion.
a. If polyatomic, just put it as it is
b. If element, change ending to -ide
If cation is a transition metal, add roman numerals.

Covalent
Between at least 2 nonmetal elements
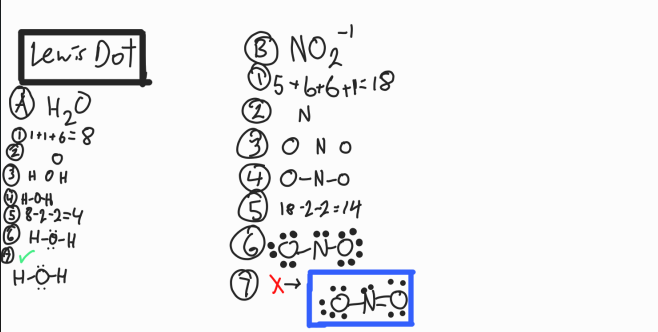
Lewis Dot Structure
Shows valence electrons, but they are shared!
Count total valence electrons
Least electronegative atom goes in the center. This is the central Atom (CN)
Place all other atoms around it symmetrically
Bond each element to CN. Each bond (—) is 2 electrons
Subtract # of electrons from total
Place remaining electrons on outer elements. Any left over go to CN
Check for octets. If there is not an octet, manipulate electrons to make it work
Notes:
Hydrogen doesn’t have octet as it can only do 1 bond
Be can do 2 bonds max
B can do 3 bonds max
C always has 4 bonds, is always the central atom, and has no lone pairs
Some elements in Period 3 and down can have bigger octets (P, S, Se, I, Xe)
O prefers double bonds
N prefers triple bonds
In bonding “:“ and “—“ are the same thing
Formula Writing
Add symbols used in name
Add numbers based on suffixes
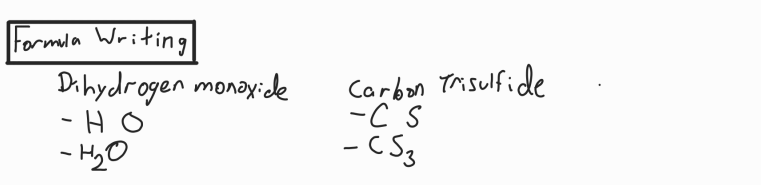
Naming Covalent
Name 1 element and add -ide to 2nd element
Add prefixes as necessary
1st element doesn’t get mono- suffix
don’t do o-o, a-o, a-a, o-a
remove vowels at the end of suffix if necessary
1—Mono 3—Tri 5—Penta 7—Hepta 9—Nona
2—Di 4—Tetra 6—Hexa 8—Octa 10—Deca
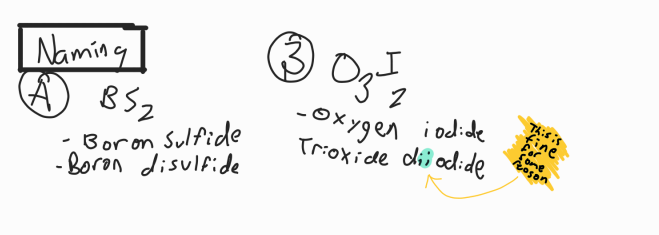
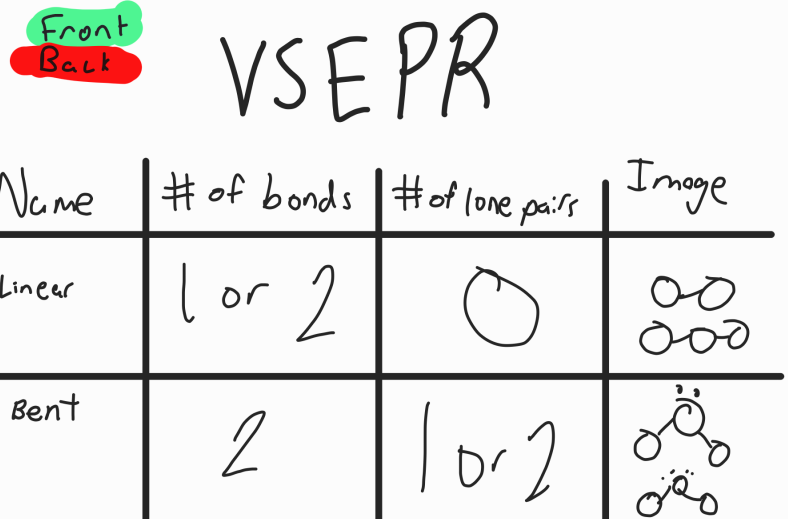
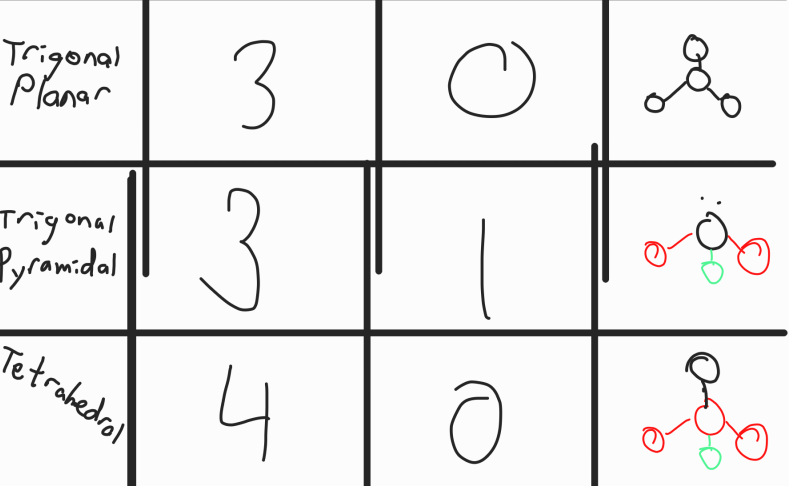
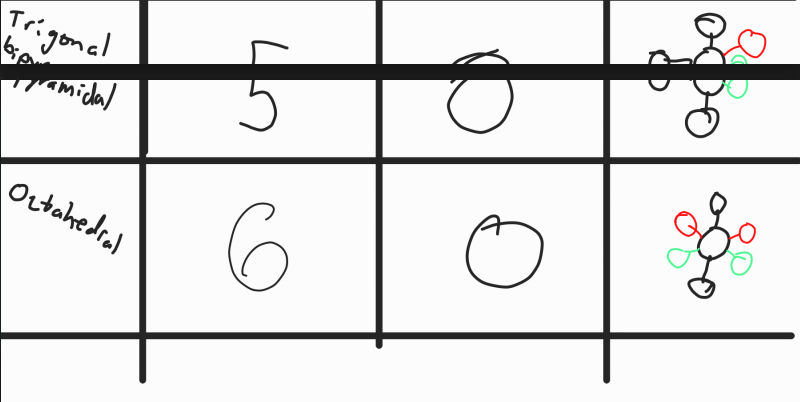
VSEPR Theory
Valence Shell Electron Pair Repulsion Theory
Predicts the shape of 3d molecules by using the fact that electrons regions (bonded pairs or lone pairs) push other electron regions away
Polarity
When there is an unequal sharing because 1 atom has a stronger charge than another side
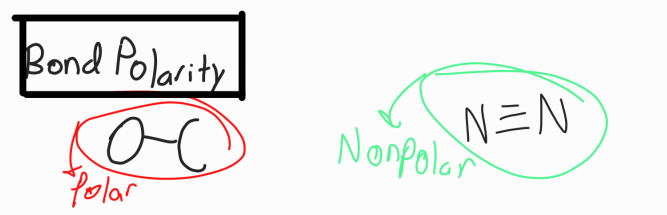
Bond Polarity
Based on EN vales
Non-polar bonds: electrons are equally shared because they are the same element
Polar bonds: electrons are unequally shared because they are different elements
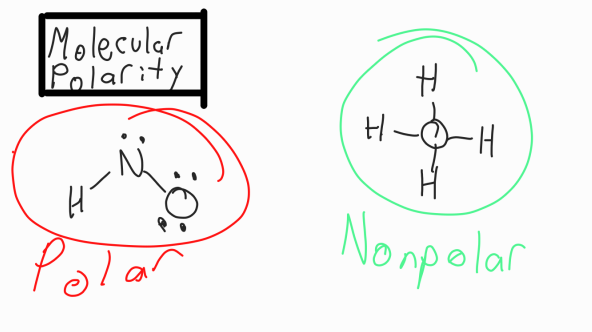
Molecular Polarity
Nonpolar:There are no lone pairs on CN AND all the elements around CN are the same
Polar: There is a lone pair on CN OR different elements surround CN