U1 P1.1
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
29 Terms
1 meter = ? inches
39.3 inches
1 kg = ? lbs
2.2 lbs
1 kg = ? grams
1000 grams
physics
the study of matter, energy and their interactions
x-ray image production
applied physics that explains the production a radiographic images (i.e. electrical energy converted to electromagnetic energy)
force acting on a body over distance (measured in joules)
work
expenditure of energy to do work (potential or actual ability)
energy
types of mechanical energy
kinetic and potential
kinetic energy
energy of motion
potential energy
stored energy
LAW of Conservation of Energy
states "that energy can neither be creatednor destroyed; rather, it can only be transformed or transferred from one form to another"
Forms of energy
light, heat, sound, chemical, nuclear, atomic, mechanical and electric
1 - battery stored energy
2 - energy released from battery
3 - light, xrays
4 - weak or strong forces within atom
5 - production of xrays
1 - chemical energy
2 - electric energy
3 - electromagnetic energy
4 - nuclear energy
5 - mechanical energy
matter
anything that has mass and takes up space and has weight
What is matter made up of?
atoms
protons give an atom
its identity --> atomic/Z#
more protons = _____ binding energy
more binding energy
neutrons and protons have the same _____ , but neutron is slights ______ than proton
mass; heavier
electrons
orbit in constant motion & spin on axis
binding energy
describes how tightly electrons are held in the energy shell
electron binding energy is
the energy required to remove the electron for the electron shell & is measured in electron volts (ev)
electron shell equation
2N²
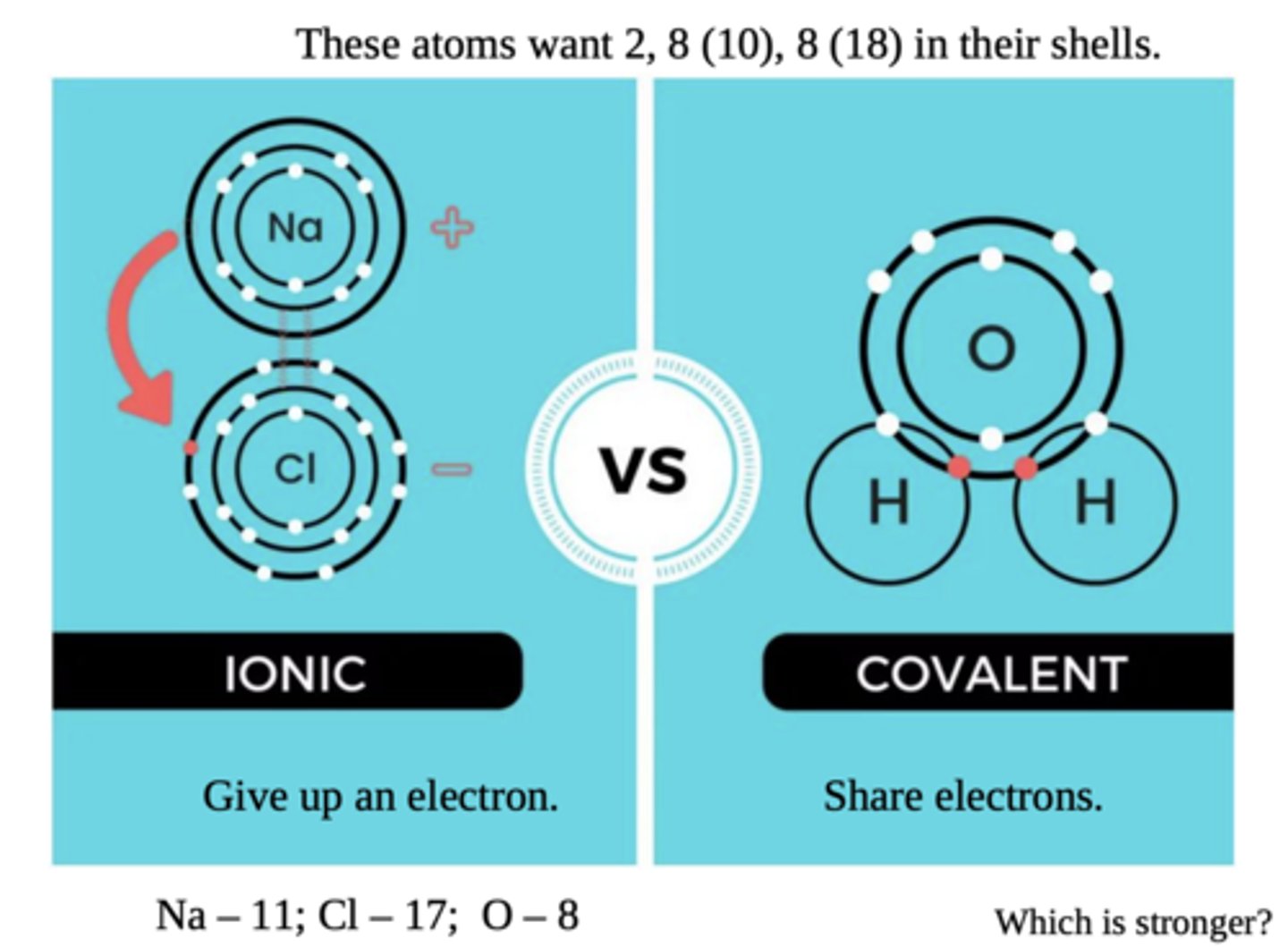
ionic vs covalent bonds
ionic: attractions of 2 opposite charges
covalent: 2 atoms sharing an orbital electron
Atomic Nomenclature
atomic/Z# = #protons
mass# = atomic weight = #protons+#neutrons
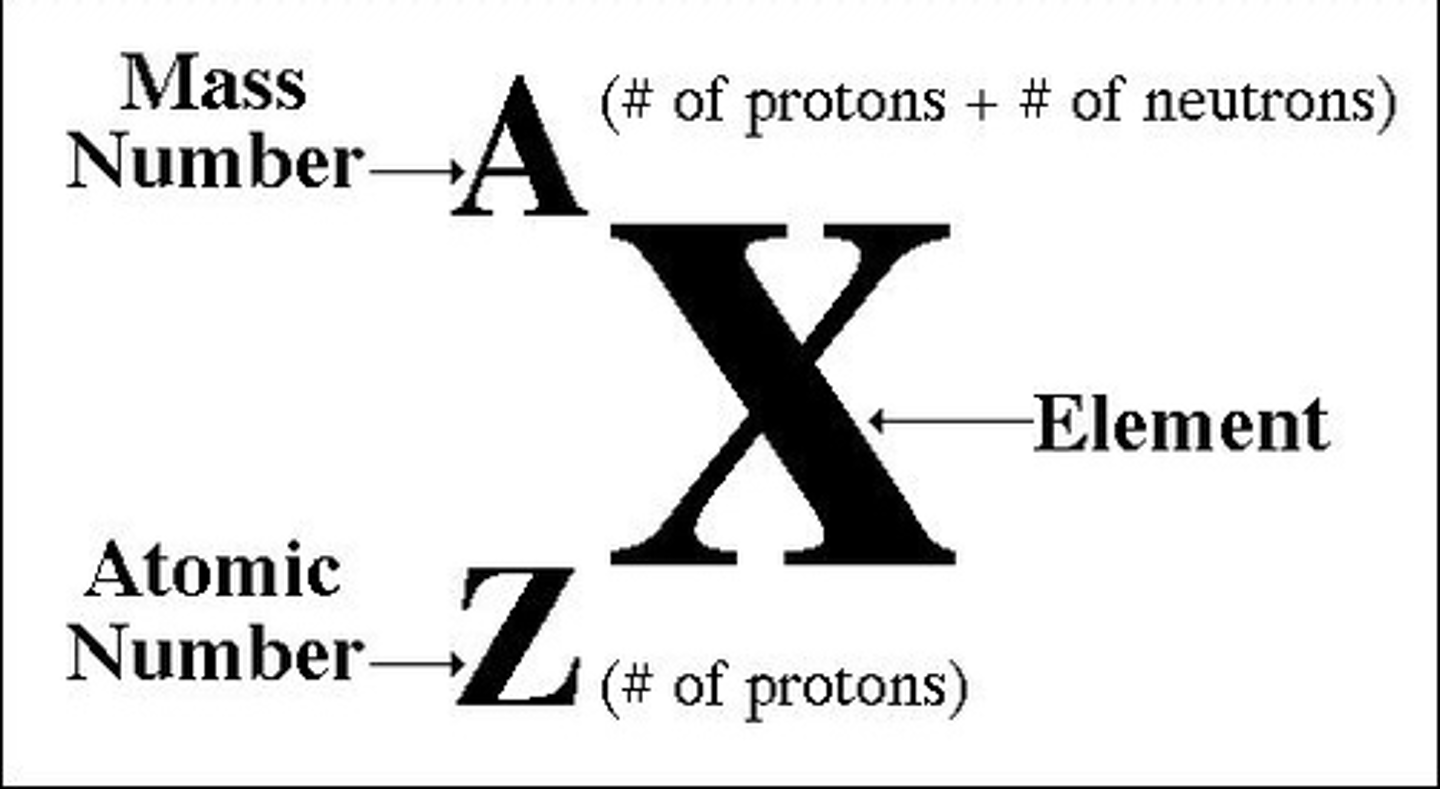
Isotopes
elements that exist in different forms; same #protons, different #neutrons & mass# (unstable-radioisotopes)
nuclide
an atom that is identified by the number of protons and neutrons in its nucleus
Ionization
the process of gain or loss of an electron from an atom or molecule
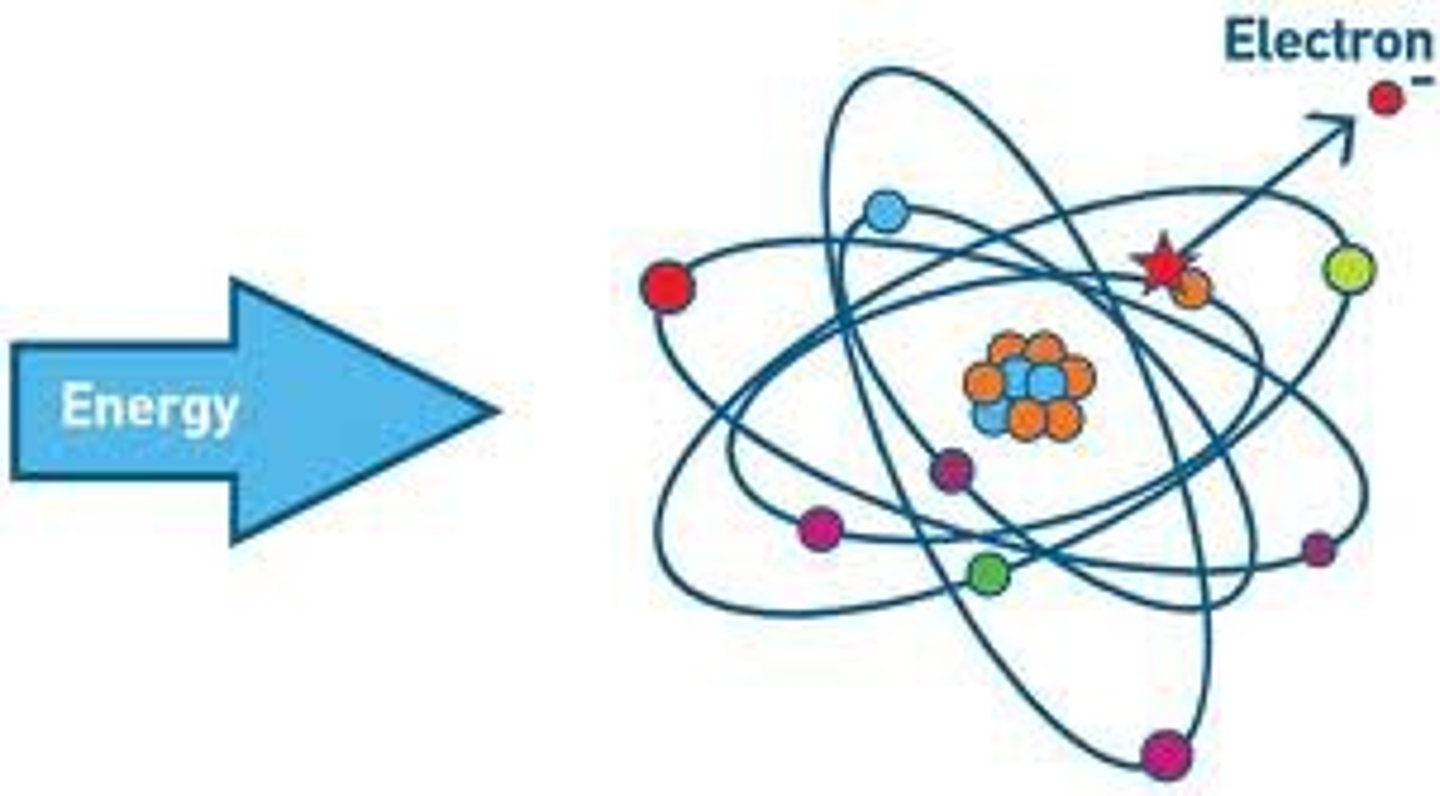
Which shell has the highest binding energy?
K shell
Which shell does the electron possess the highest kinetic energy?
outermost shell