Separating techniques: Elements compounds and mixtures: Chemistry: (9:1)
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
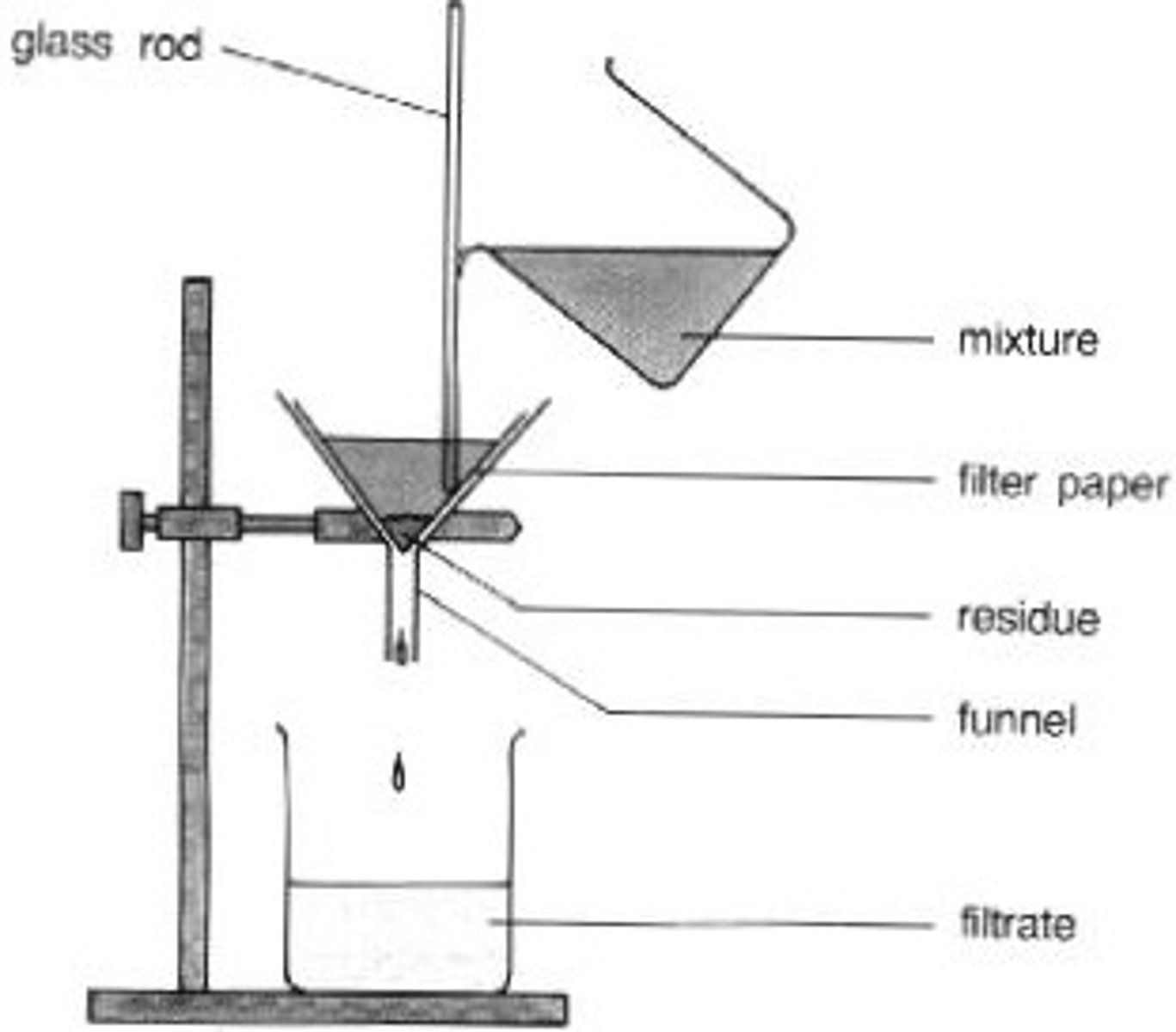
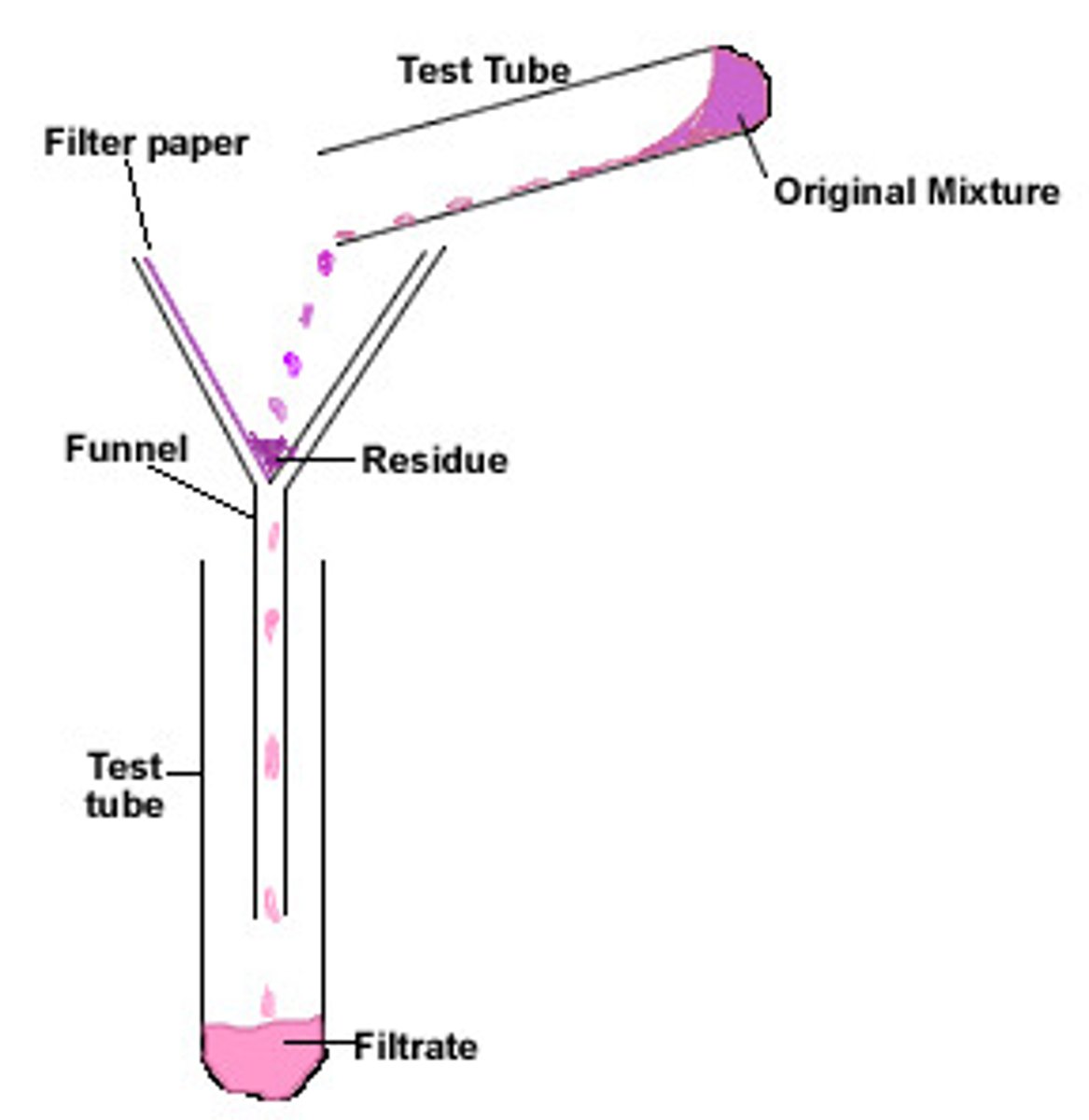
Filtration
Separates insoluble solids from liquids.
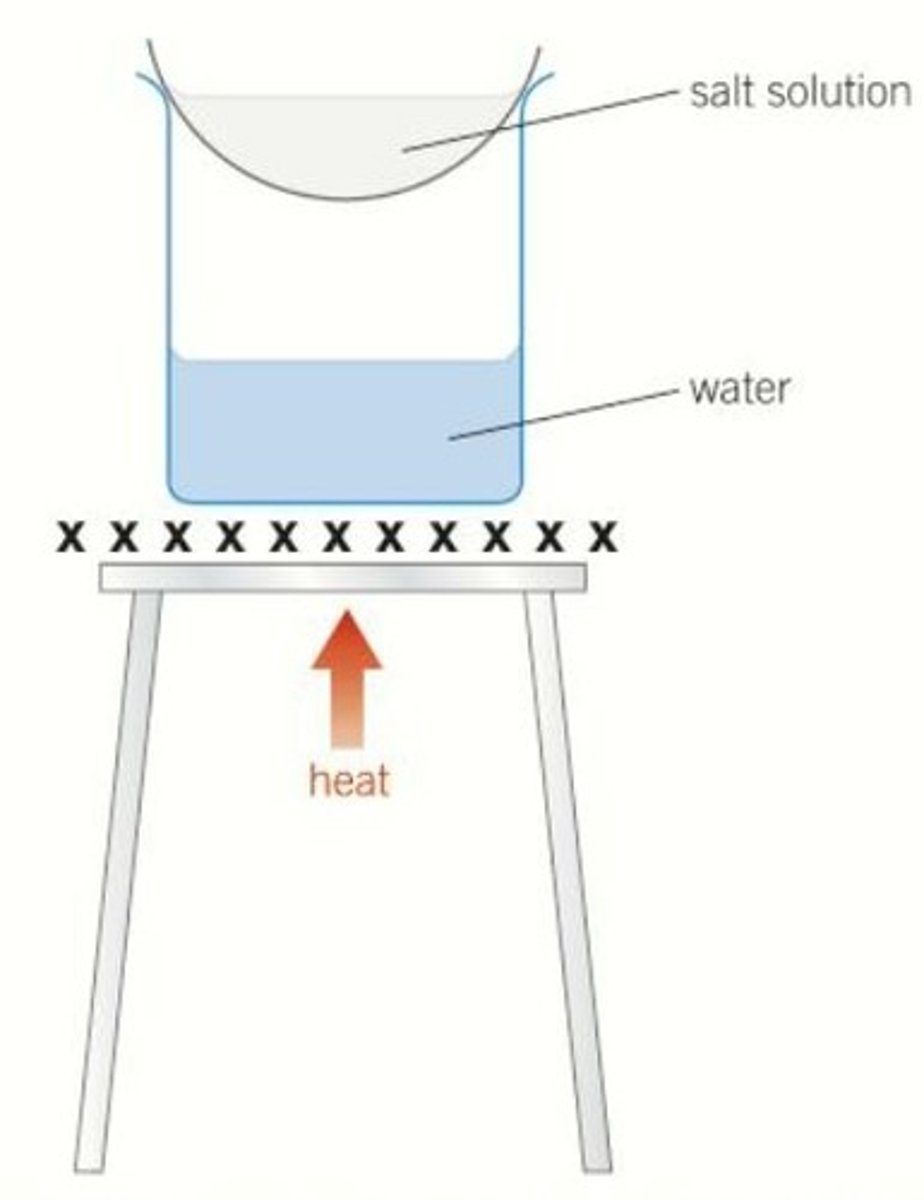
Crystallisation
The formation of crystals by cooling a saturated solution
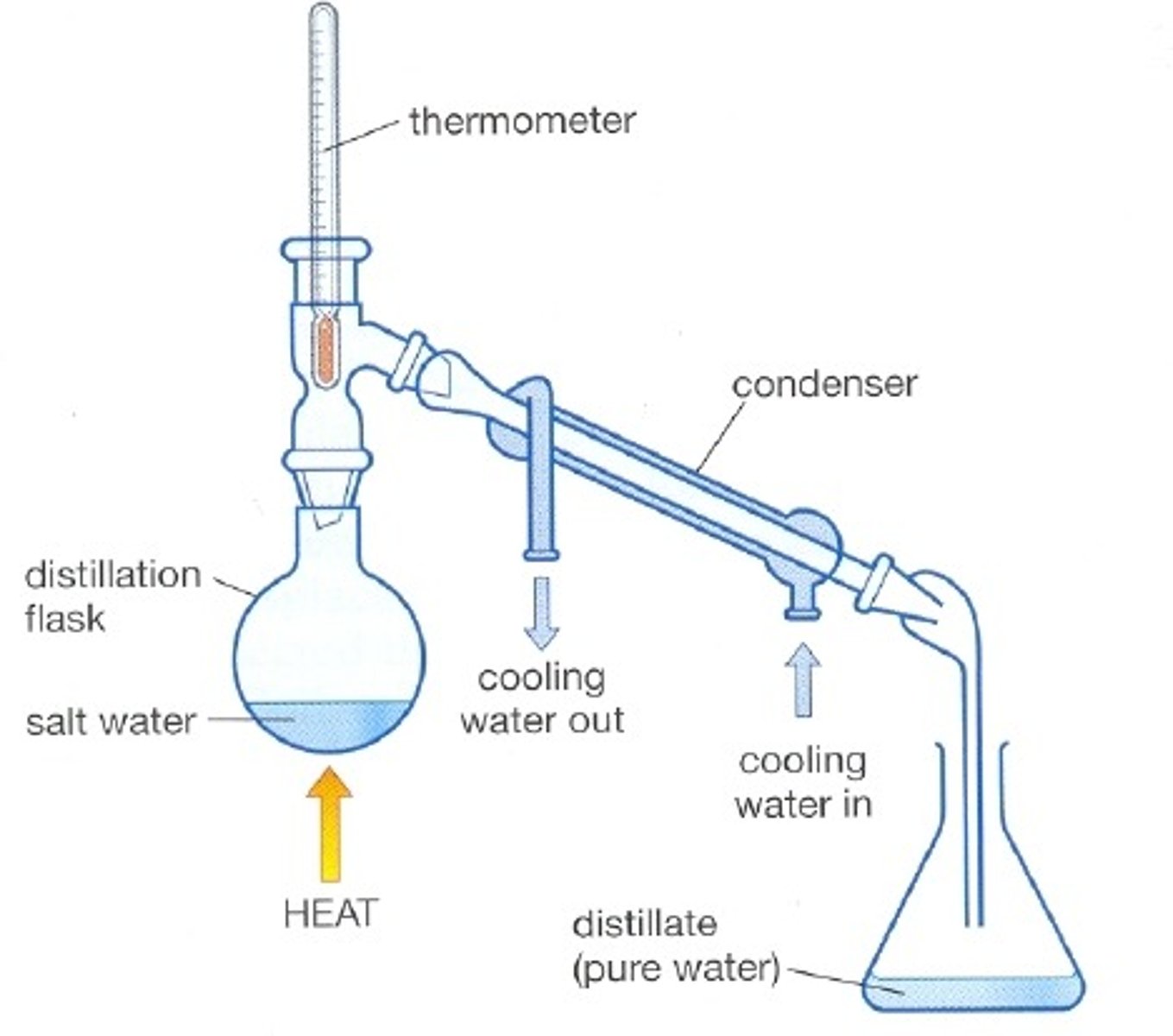
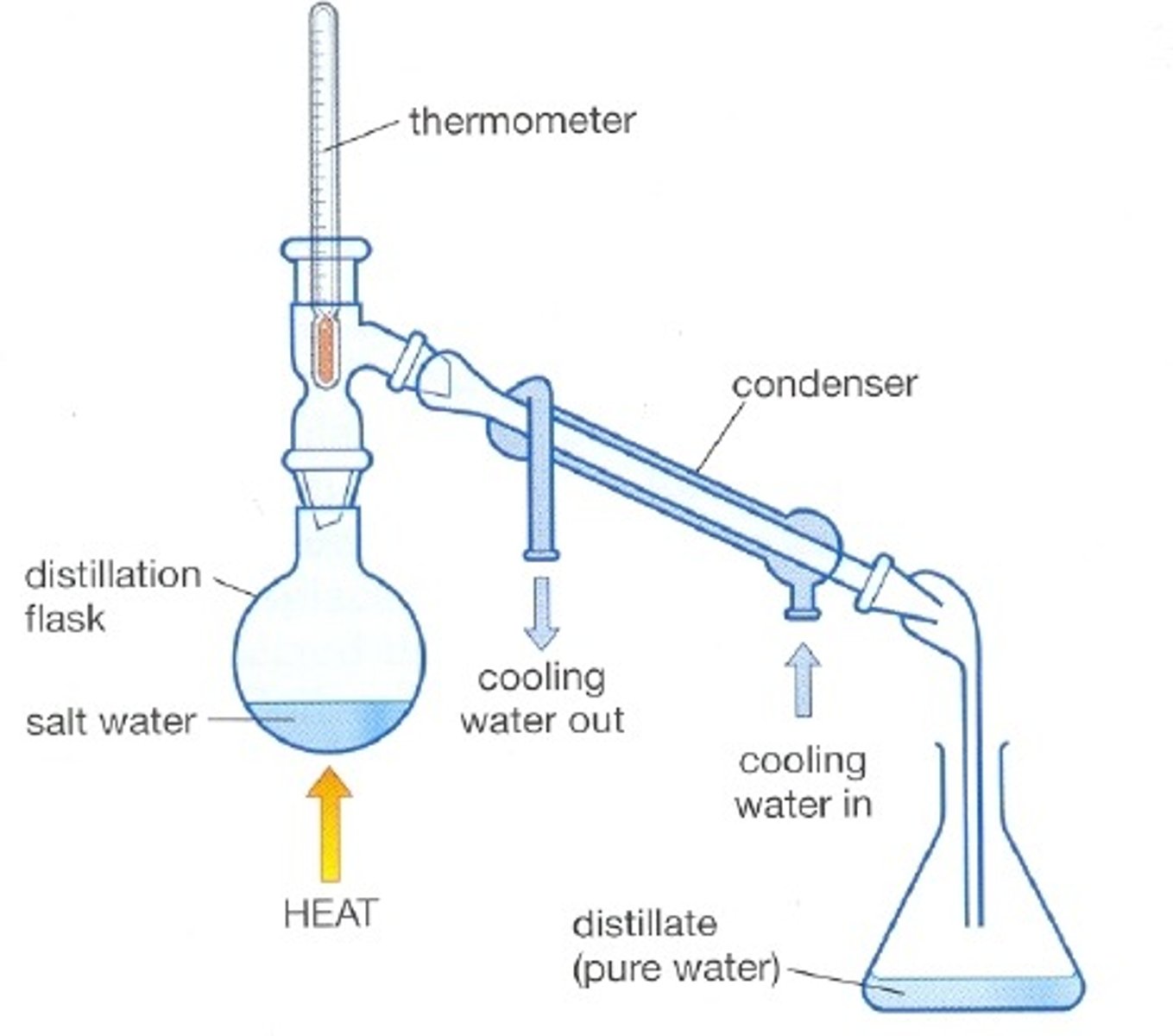
Simple distillation
Used to separate a liquid from a solution
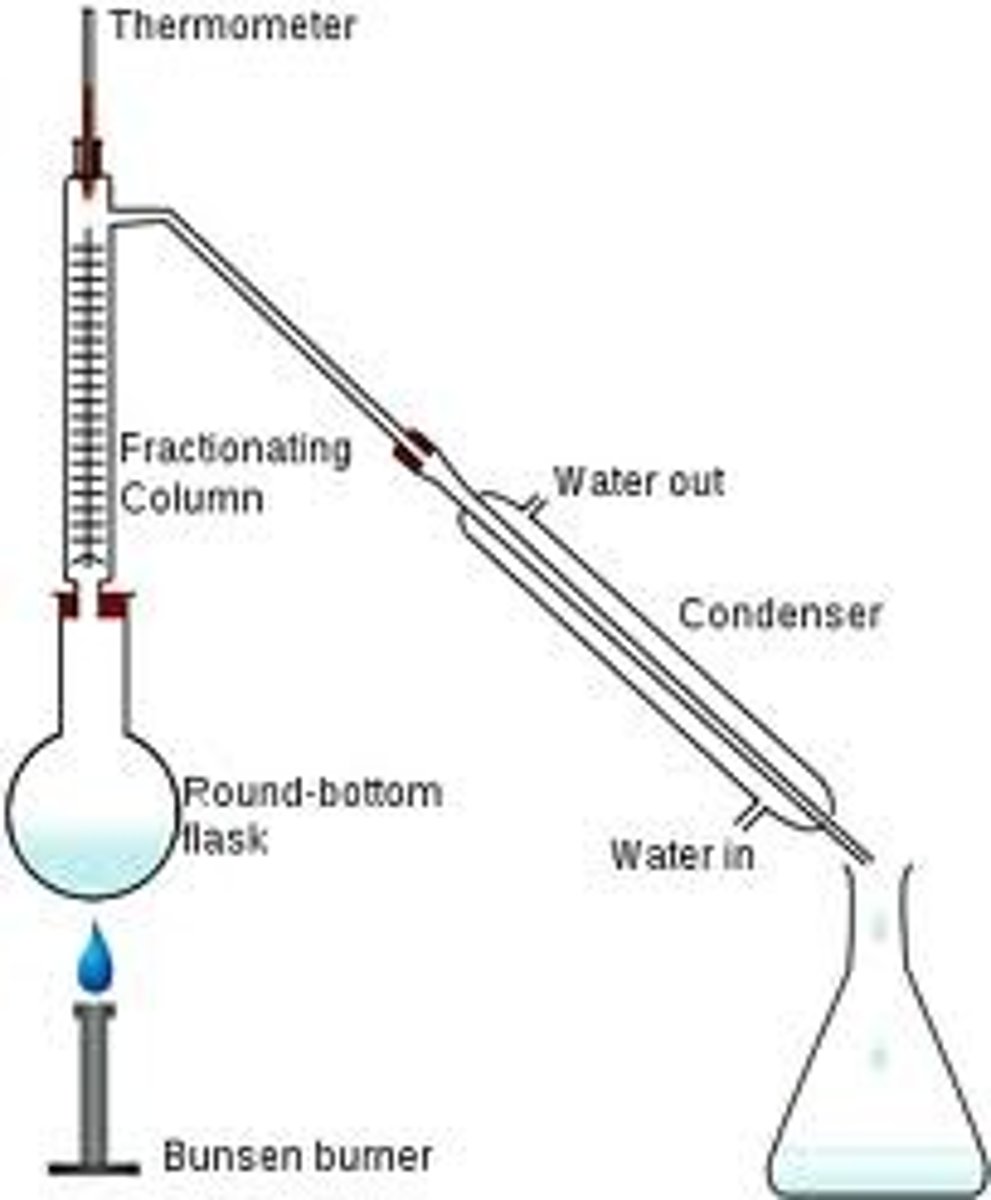
Fractional distillation
separation of a liquid mixture into fractions with different boiling points using a fractionating column.
Solution
A mixture that forms when one substance dissolves another.
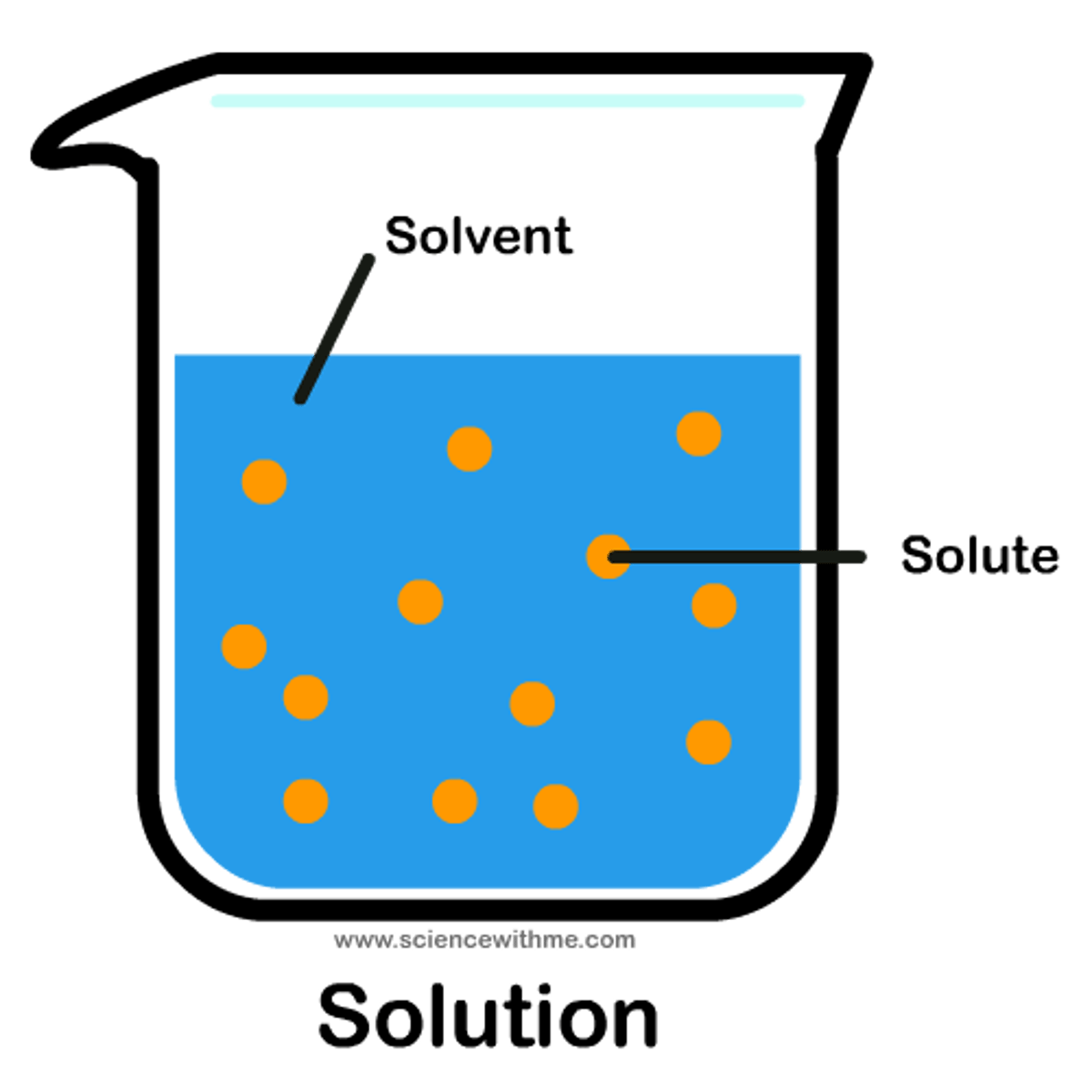
Solvent
A substance which is capable of dissolving other substances
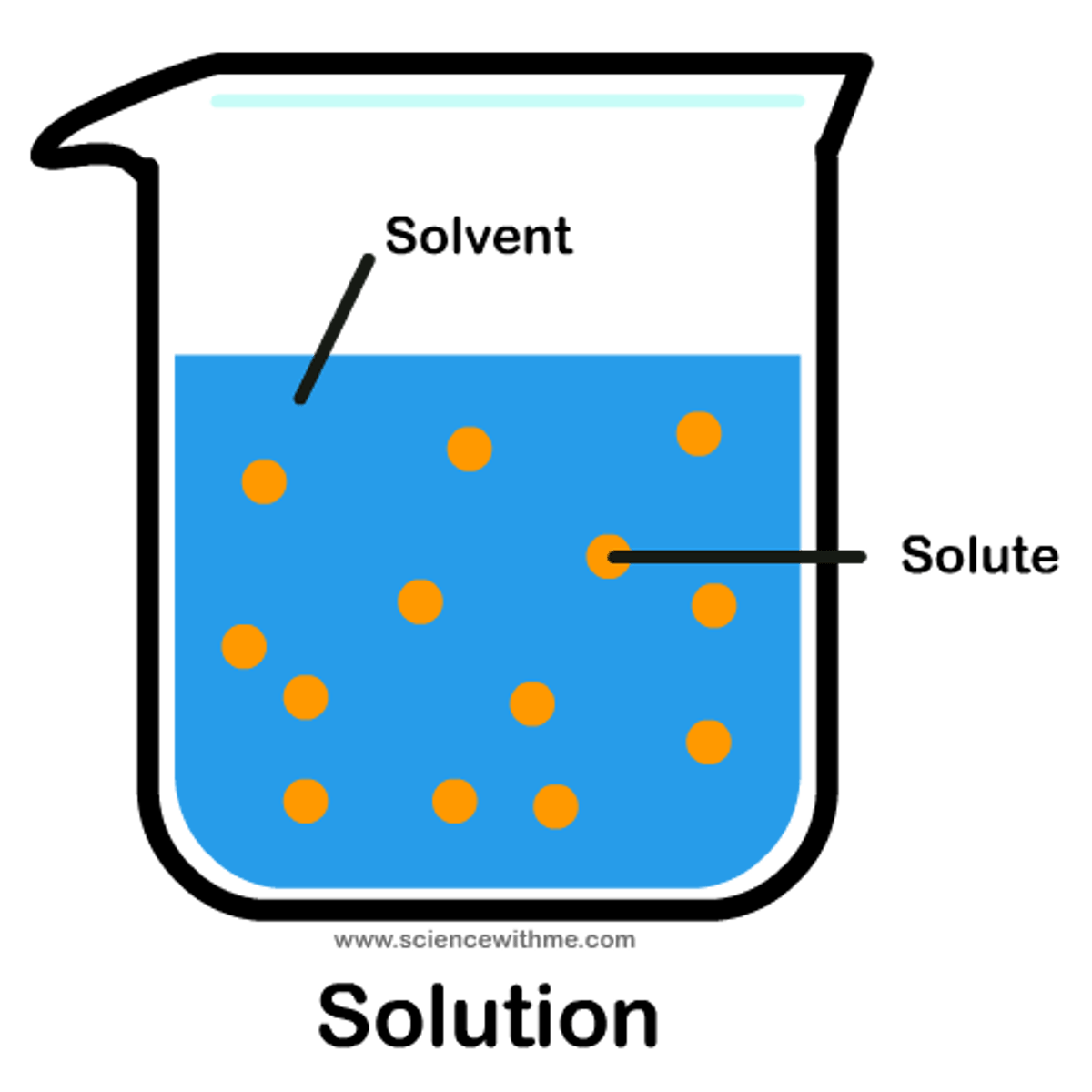
Solute
A substance that is dissolved in a solution.
Insoluble
incapable of being dissolved in a given solvent

Soluble
capable of being dissolved in a given solvent

Saturated
A solution that cannot dissolve any more solute at a given temperature

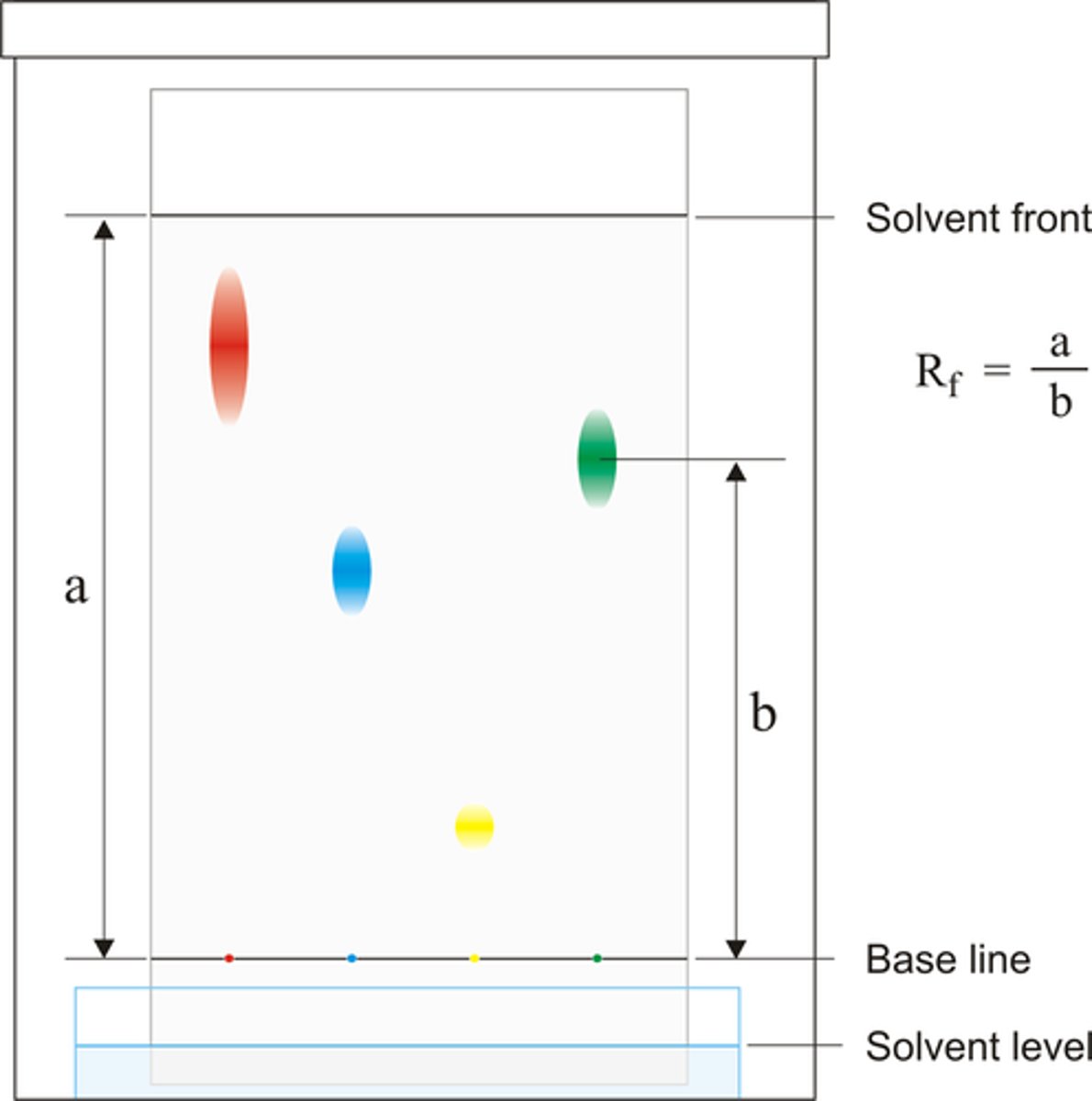
Chromatography
Separates the components of a mixture based on their solubility.
Filtrate
Liquid that has passed through a filter
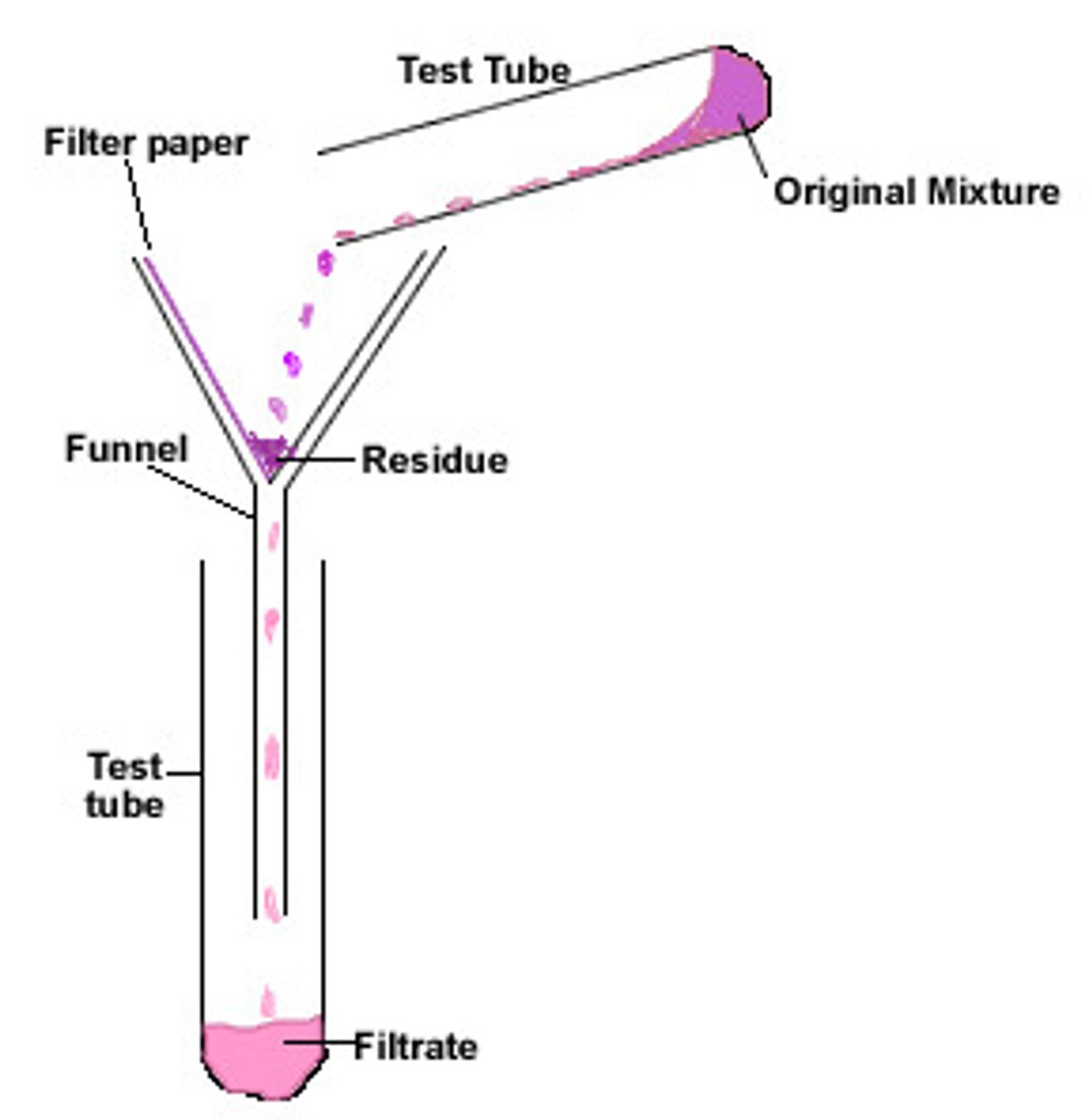
Residue
Insoluble solid which remains in the filter paper
Reason simple distillation works
Separates substances with different boiling points
Condenser
Gases pass through the tube, are cooled by the cold water and condense

First step of simple distillation
Heat the mixture so the solvent evaporates