Modern Physics Ch 2
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
48 Terms
We regard electrons as particles because they possess charge and mass we also interpret a moving electron as wave manifestation because
they exhibit diffraction, interference, and polarization
how did we find out electrons act as waves
In the double-slit experiment, electrons fired one by one still form an interference pattern — a clear sign of wave behavior
the speed 𝑐 of electromagnetic waves in free space
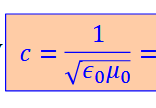
𝜖0 is …………… of free space and 𝜇0 ……………….
the electric permittivity, is magnetic permeability
electromagnetic waves travel with the speed of
light
light consists of
electromagnetic waves
X-ray frequency is from
10-8 - 10-12
destructive interference shows as , it’s wavelength is
dark line, odd number half wavelength
constructive interference shows as, it’s wavelength is?
bright line, integer number of wavelength
A Blackbody is
an ideal body that absorbs all radiation incident on it, Regardless of frequency
When metals get hot they radiate heat and light that is
EM energy
when body is in thermal equilibrium with it’s surroundings it absorbs radiation at the same rate as it……….. which walls are perfect reflectors of…………..
Emits it, standing EM waves
Formula for blackbody radiation

Oscilator energies
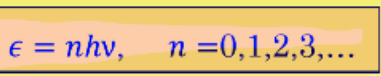
Actual average energy per standing wave
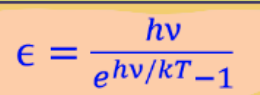
Photoelectric effect is when
we direct light at metals after freq. ν0 we see electrons emitting called Photoelectrons
under critical frequency ν …………. emit
no electrons emit
energy of Photo-electrons range form
0 to max value that increase linearly with increase in freq.
Photoelectrons acquire kinetic energy by……….. which produces ……………..that can be measured
Absorbing photon energy, Electric current
on Photoelectric effect - Higher frequency of light result in
higher energy of Photoelectron
Photon energy =
hv
in photoelectric effect work function

Photo electric formula
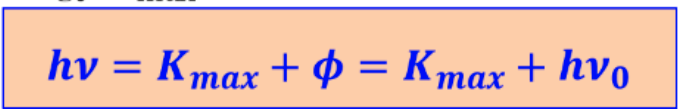
energy of incident photon can be calculated using
h*c/lamda
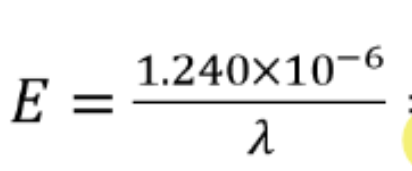
X-rays are? there wavelength range from
EM waves, 0.01 to 10 nm
in X-ray, The higher the accelerating voltage the
faster the electrons and shorter the wavelengths
X-ray production formula

in X-ray ve is Kinetic energy =
hvmax =
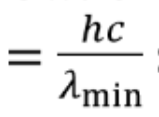
In X-ray vmax =
c/λmin
X-ray diffraction
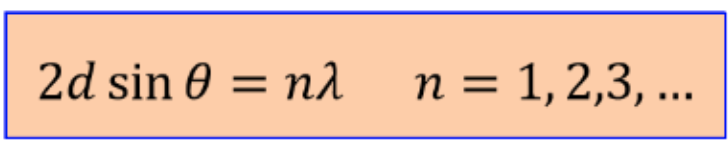
compton effect in photo
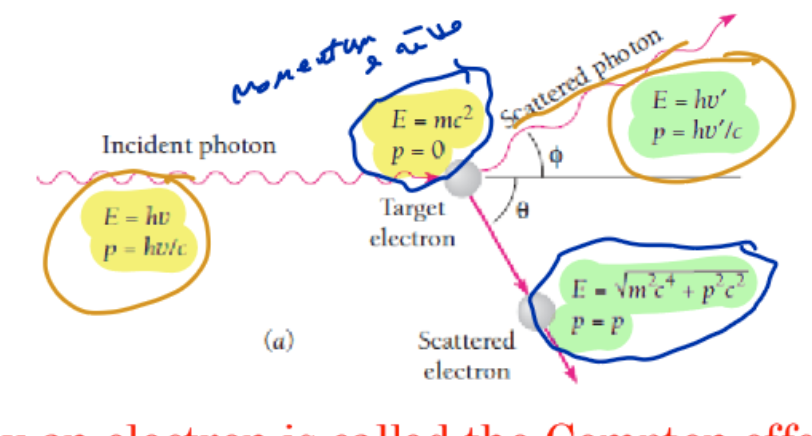
Compton effect is
scattering of electron by photon, Energy and momentum are conserved
(Compton)The photon loses an amount of its energy in the collision
which is gained by the scattered electron as kinetic energy 𝐾
(compton) scattered photon has freq. …….. than incident photon
Lower
(compton) scattered photon has……. energy and ……… wavelength
less energy, longer wavelength
Wavelength in compton
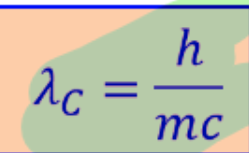
compton effect in formula
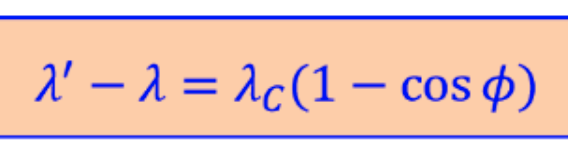
Pair production
Photon materialize into an electron and a positron
Pair production cannot occur in
empty space it needs a nucleus to conserve momenutm
Pair production need a photon of atleast
1.02 MeV any additional turns becomes kinetic energy for the electron and positron
inverse of pair production ocures when
positron ids near an electron and two become one with lost mass becoming energy in form of two gamma rays
radiation intensity is
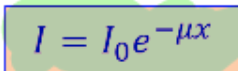
x absorber thickness is
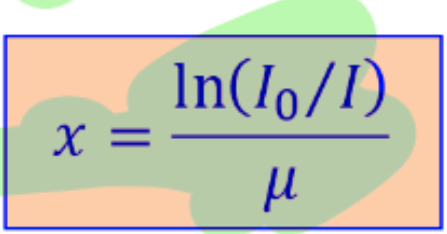
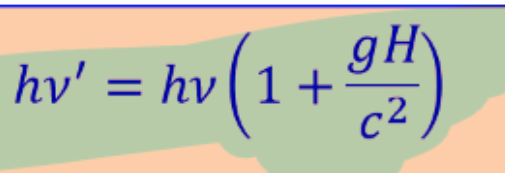
photon and gravity
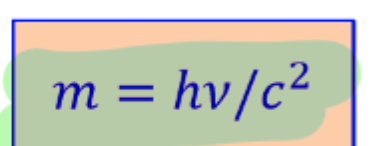
Photon energy after falling thru hight H