Chapter 5: Water Resources Quiz
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
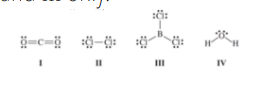
Which molecule(s) contain(s) polar covalent bonds, but is(are) nonpolar?
I and III only.
What is the molarity of sodium chloride in a solution containing 0.50 mol of sodium chloride in 500 mL of water?
1.0 M
Type the chemical name for HNO3(aq). Type the chemical name for the aqueous solution that forms when the gas HBr dissolves in water.
Nitric acid; hydrobromic acid.
Predict the products of the chemical equation: 3 LiOH + H3PO4 ’
Li3PO4 + 3 H2O
Give the name and identify the chemical formula for the ionic compound formed from the elements Zn and O.
zinc oxide
Bases are substances that increase the hydroxide ion concentration in aqueous solution. Why does ammonia (NH3), which does not contain a hydroxide group, act as a base?
Ammonia molecules remove protons from water molecules, forming hydroxide ions.
What is the concentration of hydroxide ions in an aqueous solution containing [H+] = 1 × 10−5 M?
1 x 10^-9 M
When dissolved in water, hydrogen bromide (HBr) forms hydrobromic acid. Determine the hydroxide ion concentration in a 4,500 mL solution containing 3.78 g hydrogen bromide; K = 1.00 × 10−14.
[OH-] = 9.63 × 10^−13 M
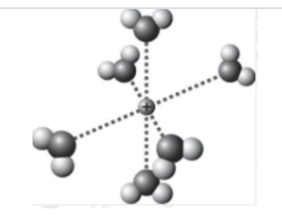
Which is the best representation showing a sodium cation in water?
A disadvantage of ozonation over chlorination is
the higher cost of ozonation and ozonation does not protect the water after the initial process is complete
Which of the following are methods of making seawater appropriate for drinking? (More than one choice may be appropriate.)
Flocculation; Reverse osmosis, Distillation.
Some types of fish can contain high levels of mercury. Which property of mercury accounts for this biomagnification?
Solubility
Which is not a form of chlorine used to disinfect water?
Chloroform
Based on the generalization in table of water solubility of ionic compounds, CaS is likely to be water-soluble
True
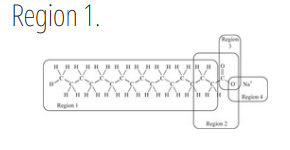
We cannot effectively clean nonpolar substances from our hands or clothing with water alone; we must add soap or detergent. The structure of a typical soap molecule is shown below. Which region of this molecule would dissolve in a nonpolar substance such as grease?
The main reason that water supplies are chlorinated is
to kill disease-causing organisms in the water
A polar covalent bond is created when
two atoms share their bonding electrons unequally
Which substance has the highest pH?
a lye solution
The fact that carbon (C) is less electronegative than nitrogen (N) means that in a C — N bond, the
shared electrons are closer to the N atom than to the C atom
Lakes surrounded by __________ have very little acid-neutralizing capacity. I. marble; II. granit; III. limestone
II only
Calculate the pH of a solution prepared by dissolving 1.2 g of potassium hydroxide (KOH) in 1,250 mL of water.
12.23
What are the major disadvantages of using ozone instead of chlorine to disinfect water?
Ozone decomposes quickly and does not provide long-term protection against possible contamination as the water is piped through a municipal distribution system.
Which correctly describes groundwater?
Any water taken from aquifers
An acidic fog in Pasadena was found to have a pH of 2.50. Which expression represents this pH measurement?
MOH− = 3.2 × 10^−12 M
Give the name and identify the chemical formula for the ionic compound formed from the elements Rb and I.
rubidium iodide
When the __________ molecules of ethanol, C2H5OH, are added to water, the ethanol molecules __________.
polar; form hydrogen bonds with the polar water molecules
Which compounds are not soluble in water at room temperature? I: BaSO4; II: PbCl2; III: KBr; IV: KNO3
I and II only
Predict the products of this reaction: KOH + HNO3
KNO3 + H2O
Evaluate the ratio MH+ (pH 3) / MH+ (pH 7).
10,000
Cranberries naturally contain benzoic acid. The chemical equation for the addition of benzoic acid to water is shown. C7H6O2 + H2O Ì C7H5O2− + H3O+ Which is the conjugate base?
C7H5O2−
Which of the following is not a consequence of biomagnification?
Lead in water can lead to brain damage in children
What is biological oxygen demand (BOD)?
a measure of the amount of dissolved oxygen microorganisms use up as they decompose organic wastes found in water
Using the patterns evident in a table of electronegativity values, Ca has a higher electronegativity relative to Br
False
The world map shows the availability of safe drinking water around the world. Which statement is true?
In the United States and Canada, more than 90 percent of the population has access to clean water
Which statement is not true?
In forming a solution, the solute dissolves other substances that are called solvents
Based on the generalization in table of water solubility of ionic compounds, LiOH is likely to be water-soluble.
True
Based on the generalization in table of water solubility of ionic compounds, CaCO3 is likely to be water-soluble.
False
Based on the generalization in table of water solubility of ionic compounds, PbSO4 is likely to be water-soluble.
False
Type the chemical name for LiOH.
lithium hydroxide
Which is not a consequence of hydrogen bonding between water molecules?
Water is colorless and odorless