A2 Inorganic Chemistry
1/87
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
88 Terms
Transition element
Element which forms stable ions with a partially filled d orbital
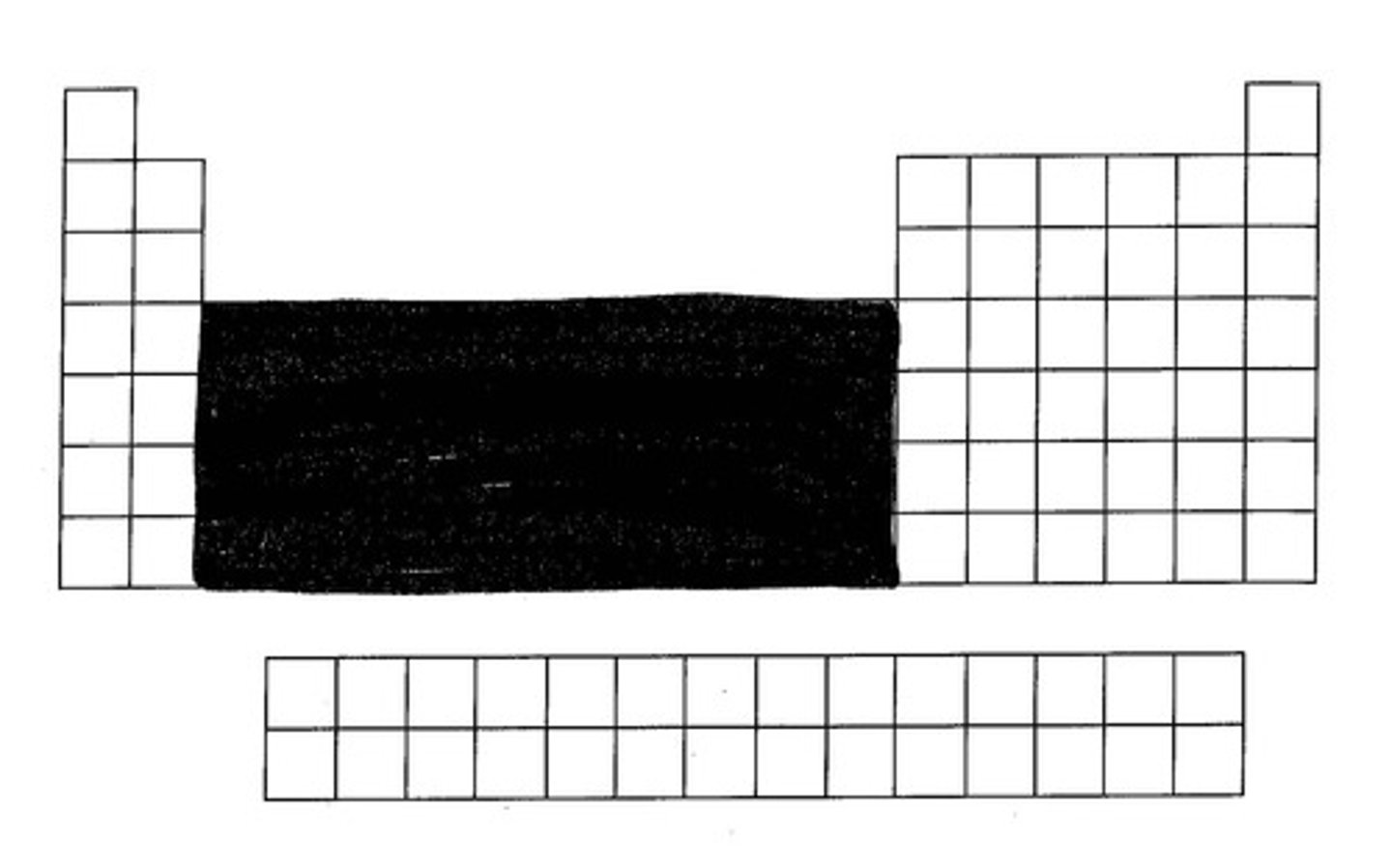
Are Sc and Zn transition elements?
No - they don't form stable ions with partially filled d orbital (only empty/full)
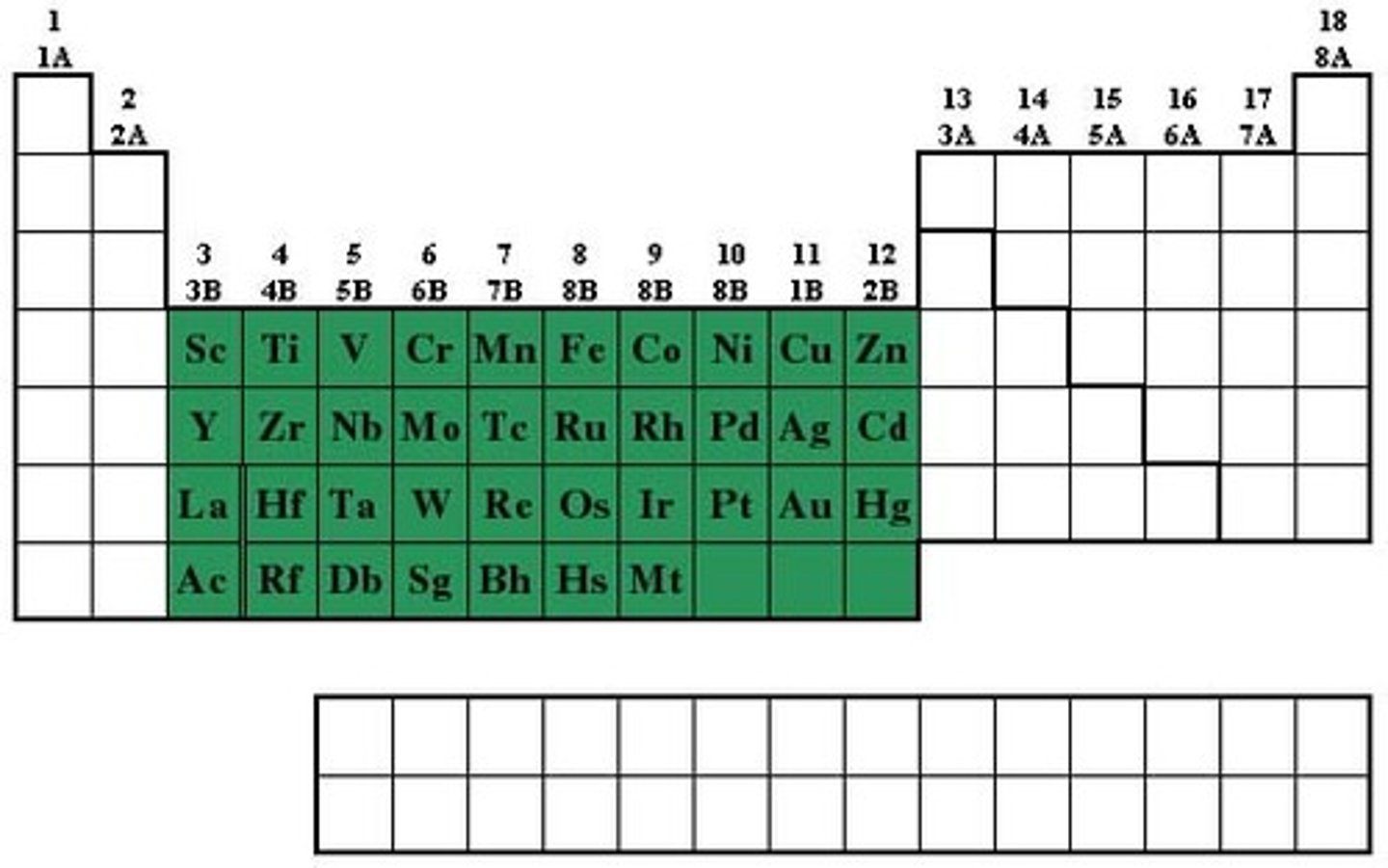
Chemical properties of Transition elements
- Variable oxidation states (does redox)
- Catalysts
- Form coloured ions
- Form complex ions

Complex ion
Central metal atom/ion surrounded by coordinately bonded ligands
Ligand
Molecule/ion that forms a co-ordinate bond with transition metal by donating a pair of electrons
Types of ligands
Monodentate - forms 1 coordinate bond (eg H₂O, Cl, CN, NH₃)
Bidentate - forms 2 coordinate bonds (donates 2LP)
Multidentate - forms more than 1 coordinate bond
Ethanedioate ion
2-
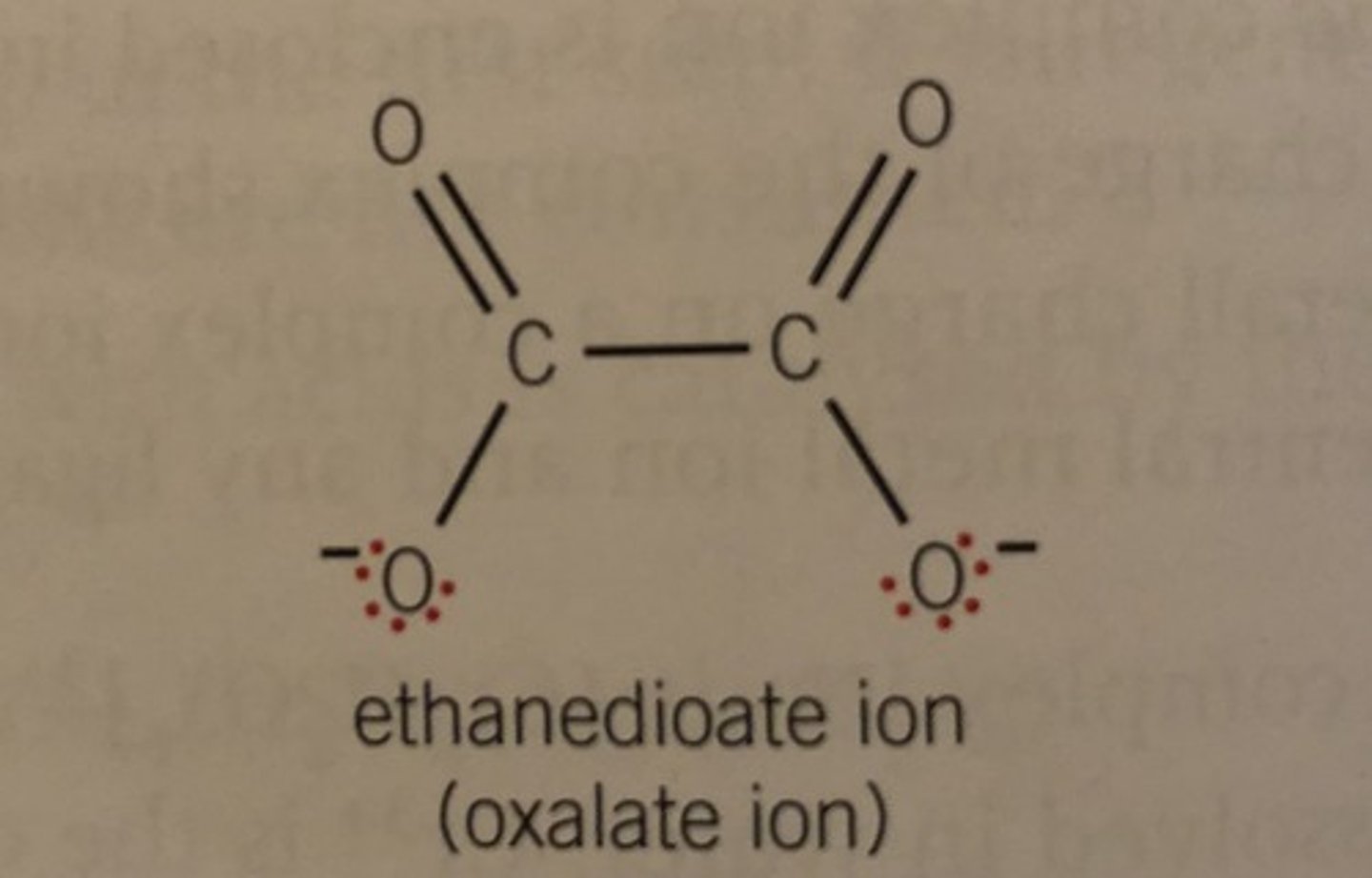
How many coordinate bonds does EDTA (multidentate) form?
6
How many small ligands (H₂O, NH₃) can fit around the central metal ion?
6
How many larger ligands (Cl⁻) can fit around the central metal ion?
4
What are the main shapes of complexes?
Octahedral, tetrahedral, square planar
Which complex has a linear shape?
Ag+ complex ([Ag(NH₃)₂]+)
![<p>Ag+ complex ([Ag(NH₃)₂]+)</p>](https://knowt-user-attachments.s3.amazonaws.com/34ac2b44-5712-4450-82e1-262c0844162c.png)
What affects complex shape?
- Size of ligands
- Co ordination number
Co ordination number
Number of co-ordinate bonds from ligands to metal ions
How to calculate oxidation state of metal in complex?
Total oxidation state - Total oxidation state of ligands
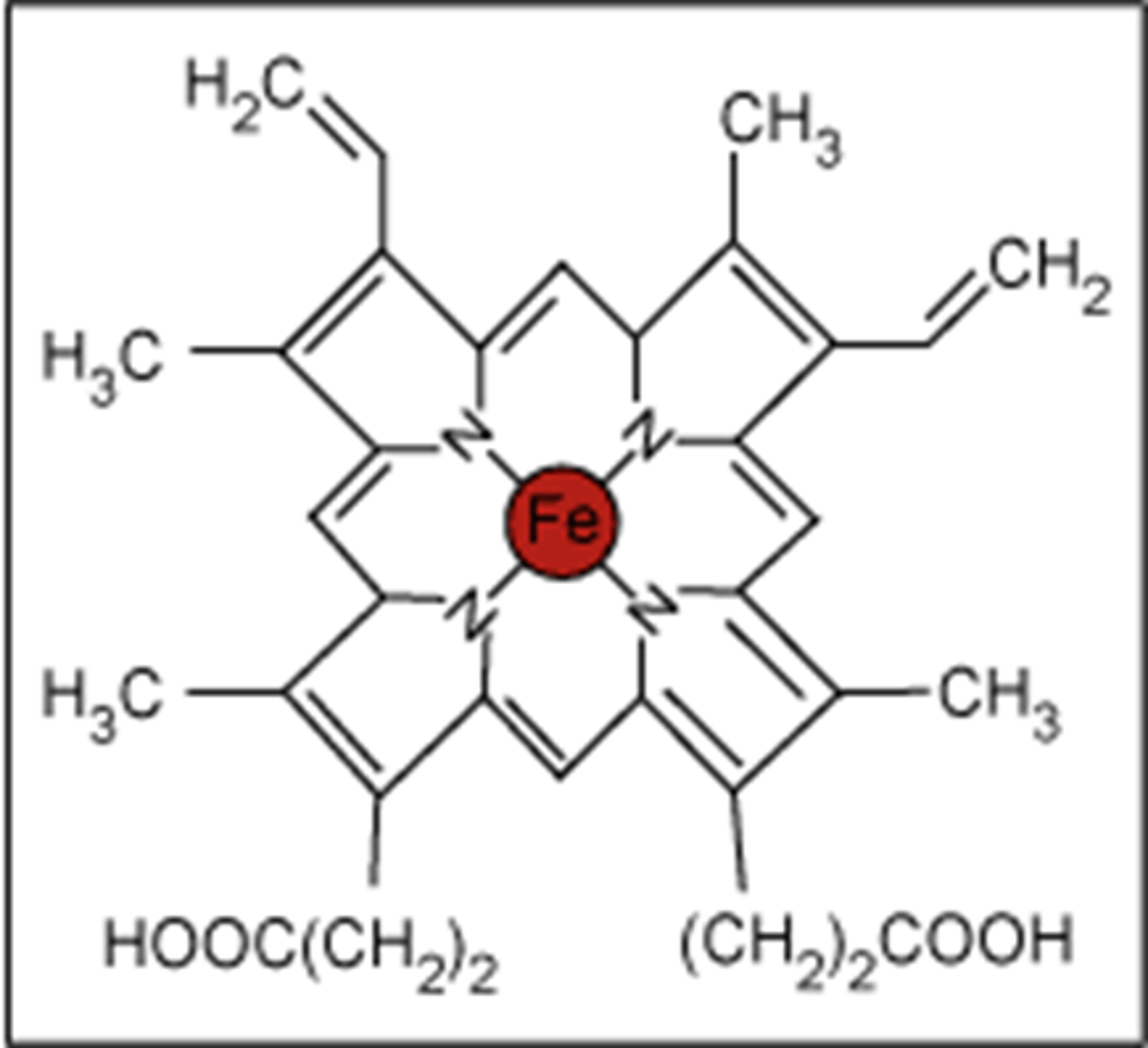
What is Haem?
An Fe²⁺ complex with a multidentate ligand
How does haemoglobin transport oxygen?
- O₂ forms weak co-ordinate bond to Fe²⁺ in Hb
- O₂ replaces water ligand and is transported
- Bond breaks when O₂ is released in cells and water replaces it
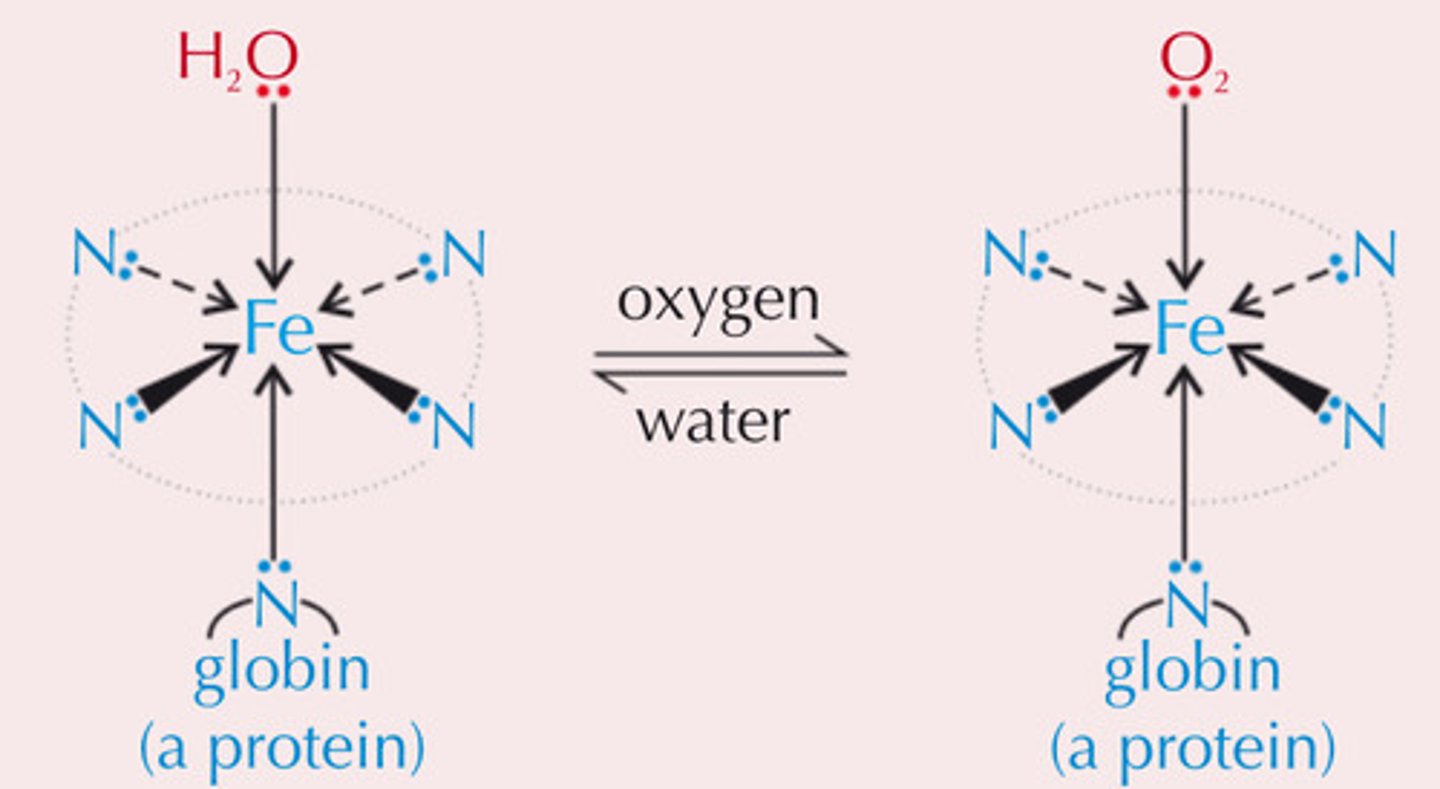
Why is CO toxic?
- CO is a better ligand and forms stronger bonds with Fe²⁺ (less easily replaced)
- Stops O₂ from bonding to Hb so it can't be transported around body
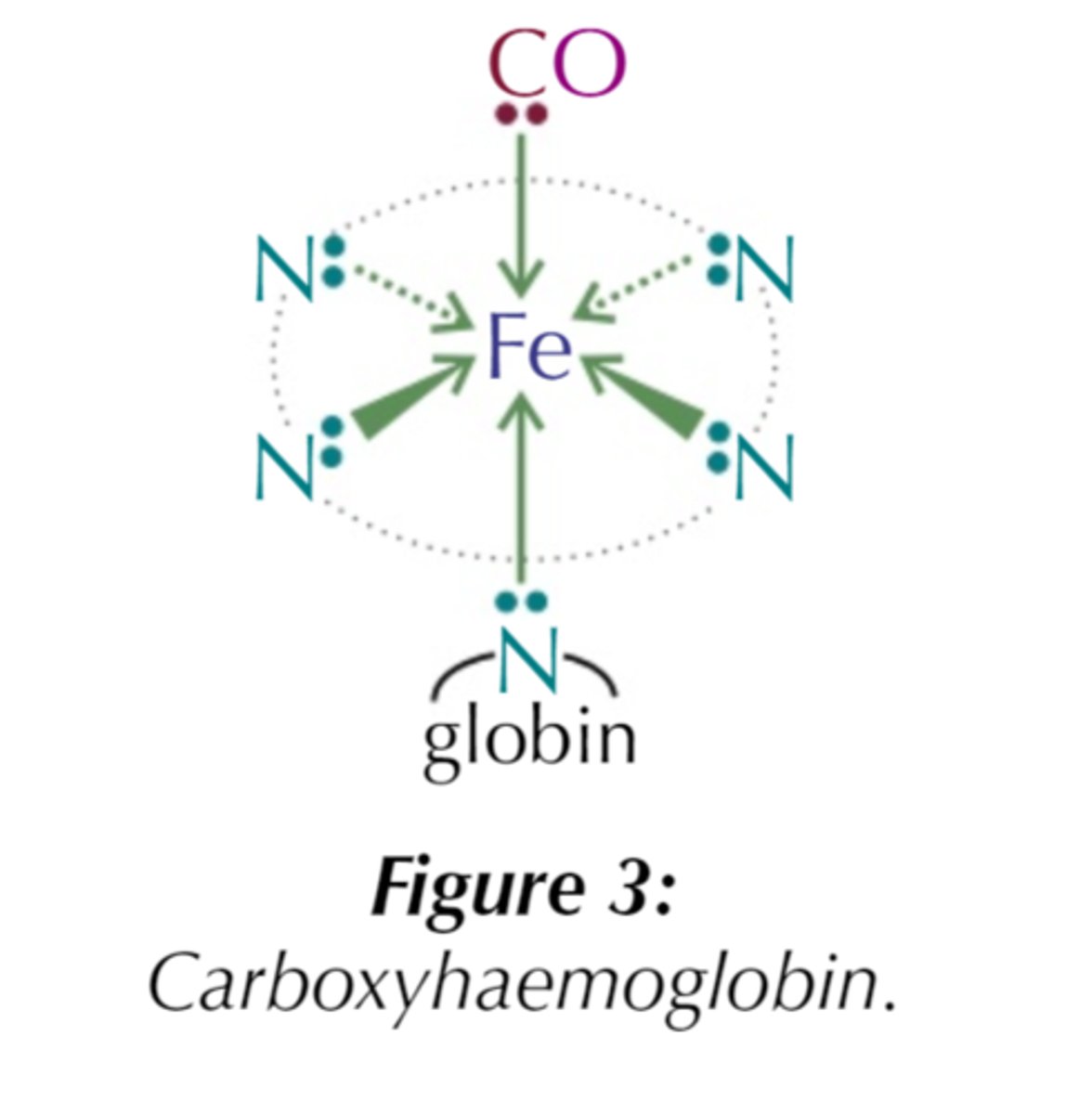
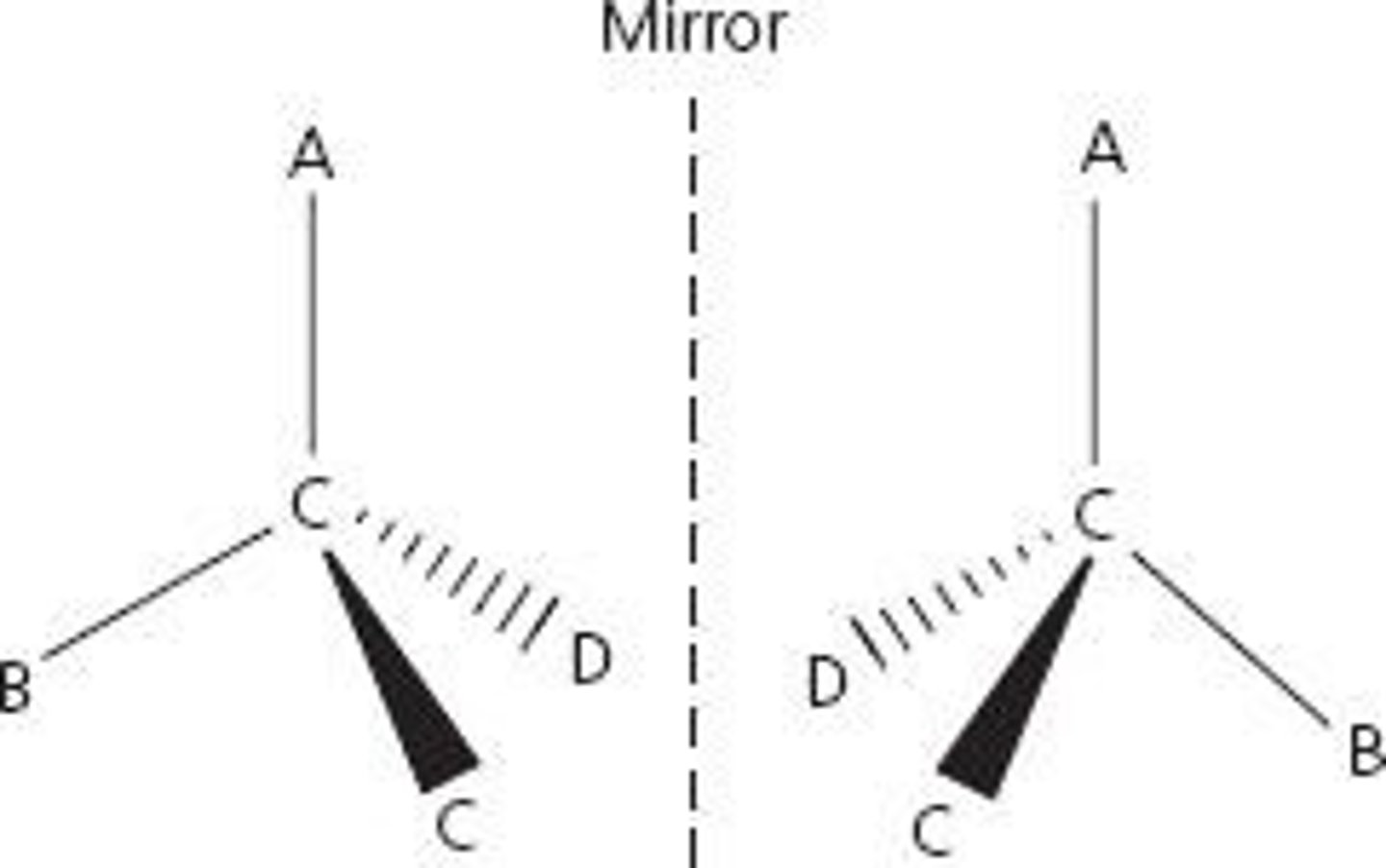
What type of isomerism can octahedral display with bidentate ligands?
Optical isomerism
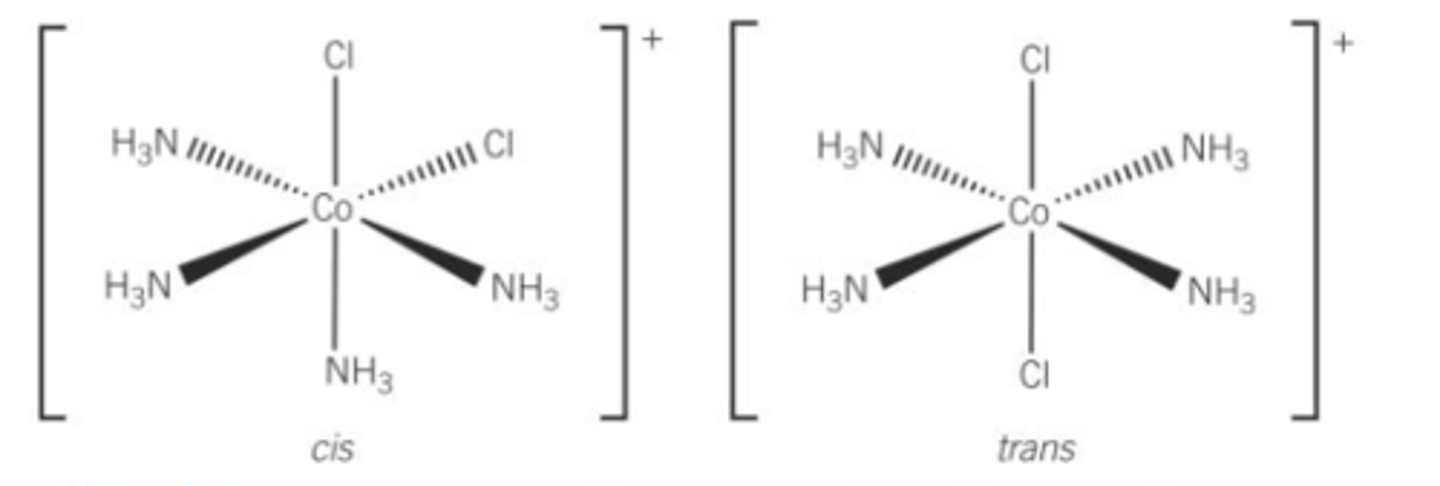
What type of isomerism can octahedral display with monodentate ligands?
Cis(Z)/Trans(E) isomerism
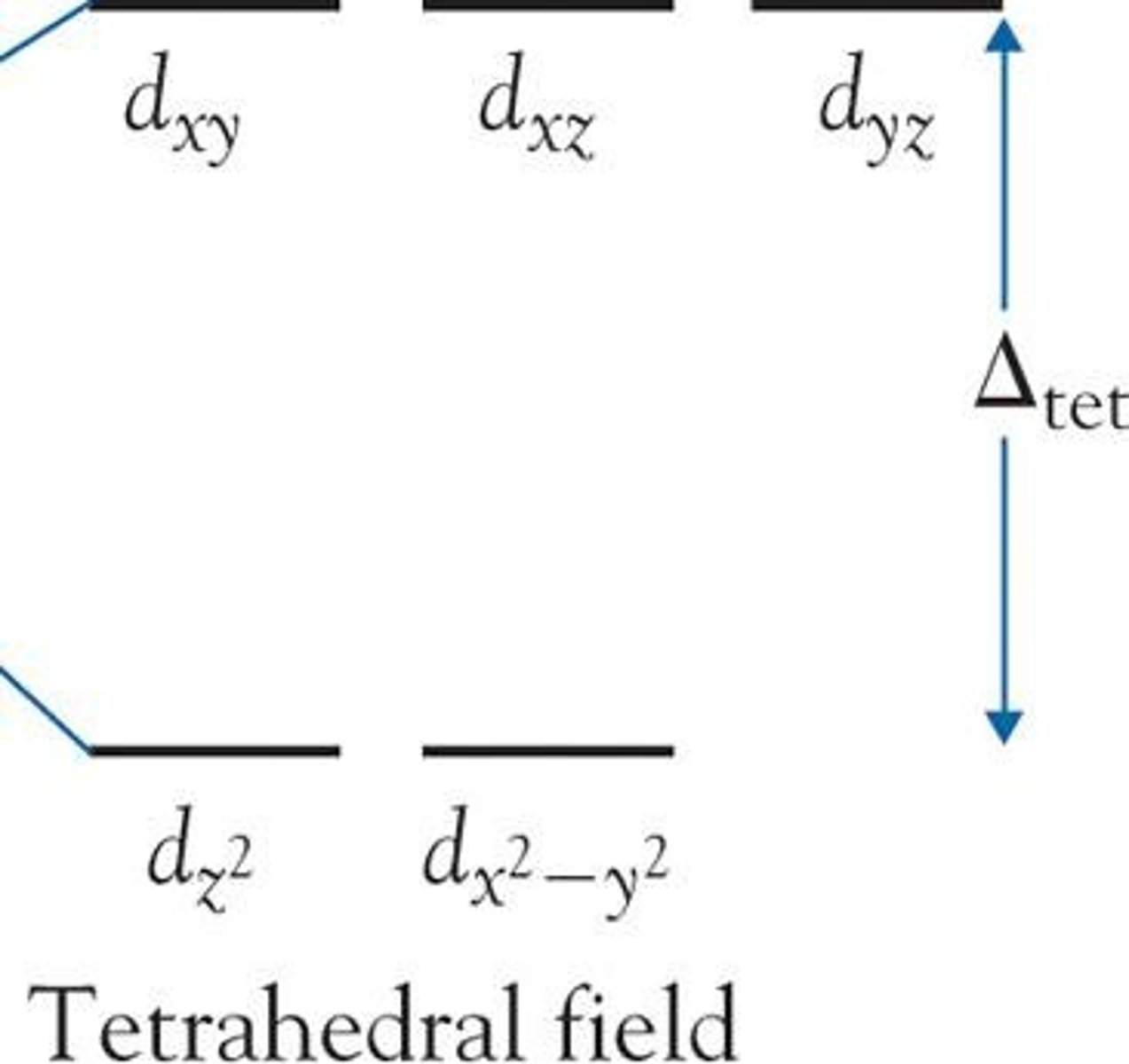
What is d orbital splitting?
d orbital splits into 2 energy levels (ground and excited state) when ligands bond with central metal ion
What affects ΔE?
- The transition metal
- Oxidation state of ion
- Type of ligands
- Coordination number
How to calculate energy absorbed by electrons?
h = Planck's constant
v = frequency of light absorbed
c = speed of light
λ = wavelength
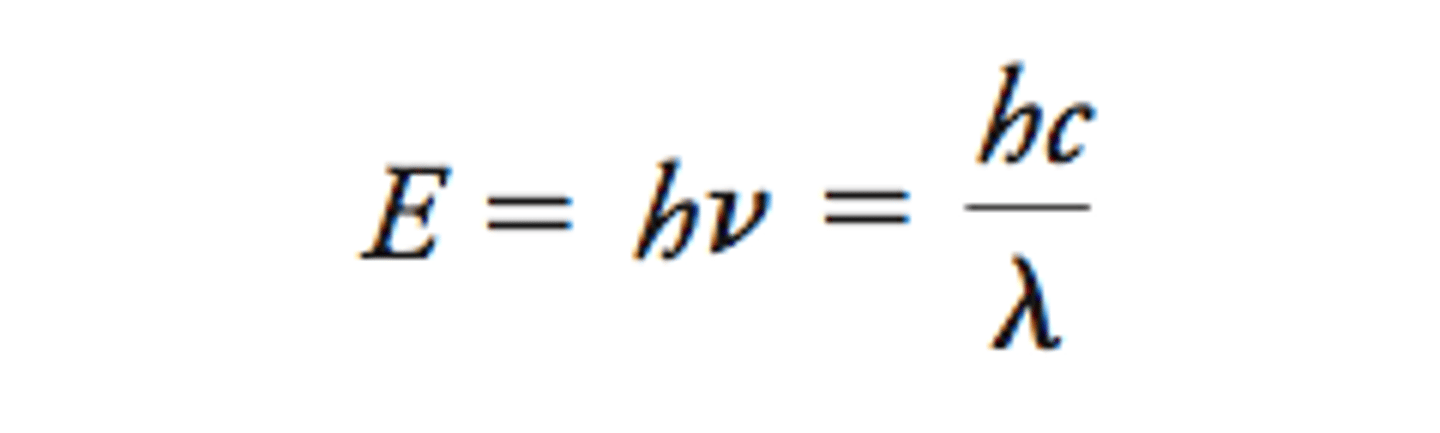
What affects the colour of transition metal compounds?
ΔE affects the frequency of light absorbed
Colour of Sc and Zn
Colourless/white - full/empty d orbital so no space for electrons to migrate

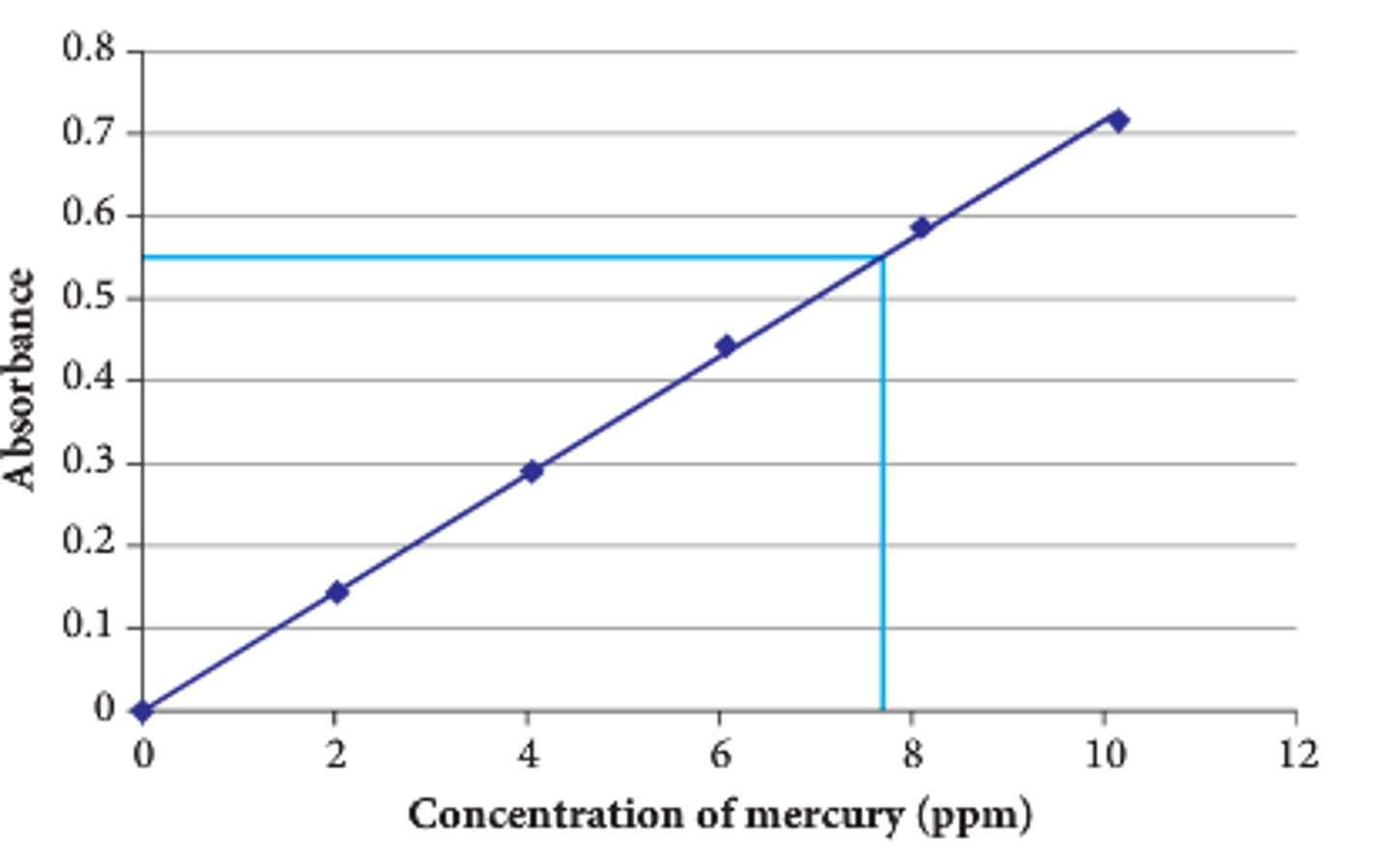
How can we measure concentration of coloured transition metal solutions?
- Use colorimetry
- Measure absorbance for a range of known concentrations
- Plot a graph of absorbance vs concentration (calibration curve)
- Measure absorbance of coloured complex
- Find its concentration from the graph
How are transition metals coloured?
- White light is a spectrum of wavelengths
- E⁻ s absorb some wavelengths of light (=ΔE)
- E⁻ s become excited and move to higher orbital
- Remaining wavelengths are transmitted/reflected by the complex
- Only transmitted/reflected wavelengths combine to form colour we see

What is ligand substitution?
One ligand is swapped for another causing colour change
What happens in substitution involving NH₃ and H₂O?
Coordination number and size doesn't change - due to similar size and charge
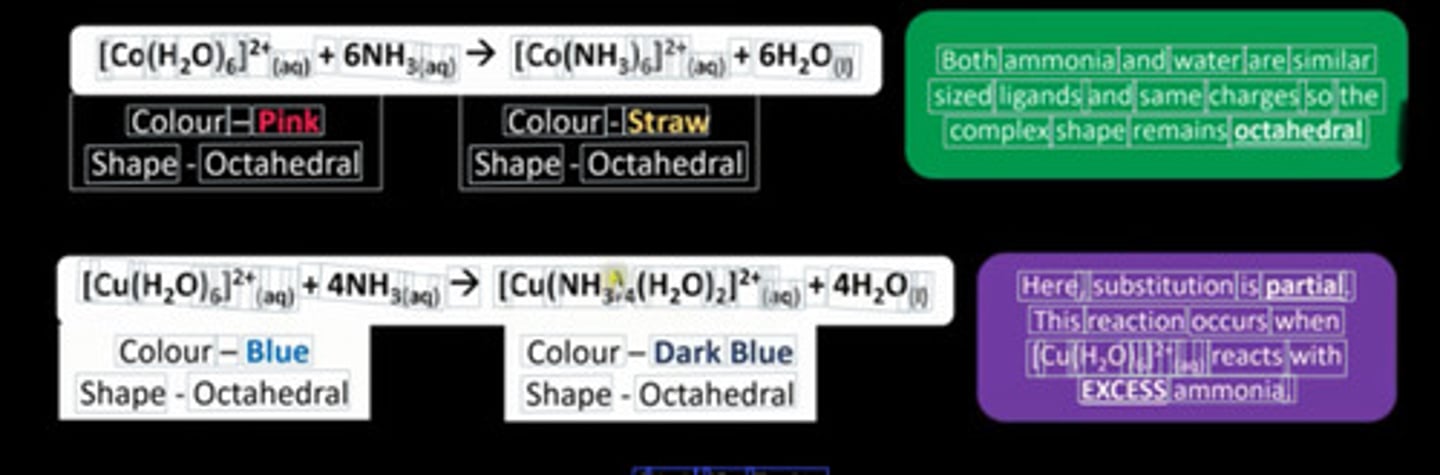
Example of partial substitution
When [Cu(H₂O)₂]²⁺ reacts with excess ligand (ammonia)
![<p>When [Cu(H₂O)₂]²⁺ reacts with excess ligand (ammonia)</p>](https://knowt-user-attachments.s3.amazonaws.com/b18f91a4-1f47-4a04-a024-92ac9edd87dd.png)
What happens in substitution involving Cl⁻?
Change in shape and coordination number
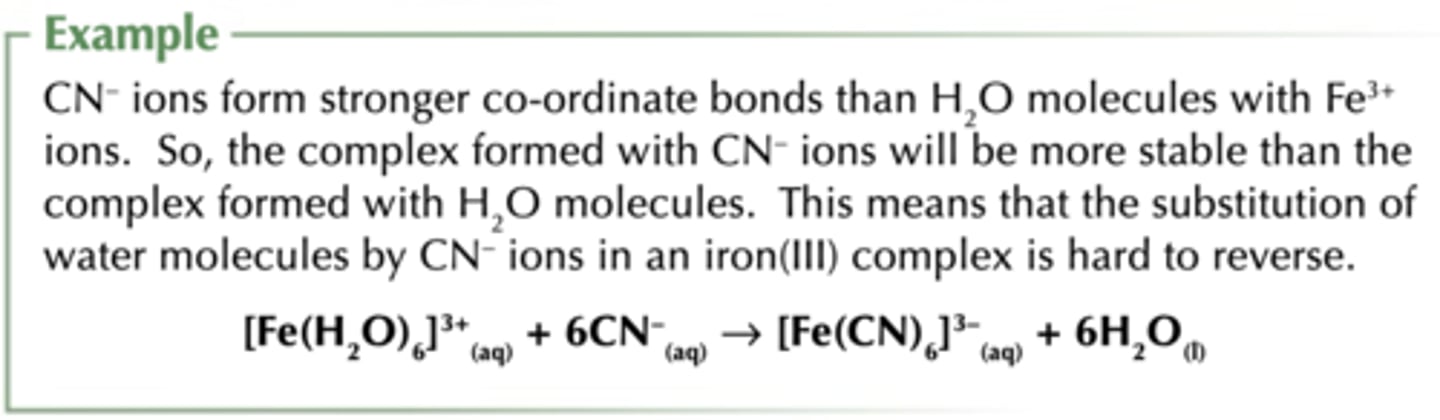
Ligand substitution reactions can be reversed unless...
New complex ion is much more stable than old one
What increases complex stability?
- Ligands that form stronger bonds with the central metal ion
Eg; CN- forms stronger bonds with Fe ions than H2O
- Replacing monodentate ligands with multidentate ligands
Is the enthalpy change for a ligand substitution reaction, big or small?
Small - strength of co-ordinate bonds forming and breaking is similar
Chelate effect
Multidentate ligands replace monodentate ligands
- Increases number of particles
- Increases entropy and stability
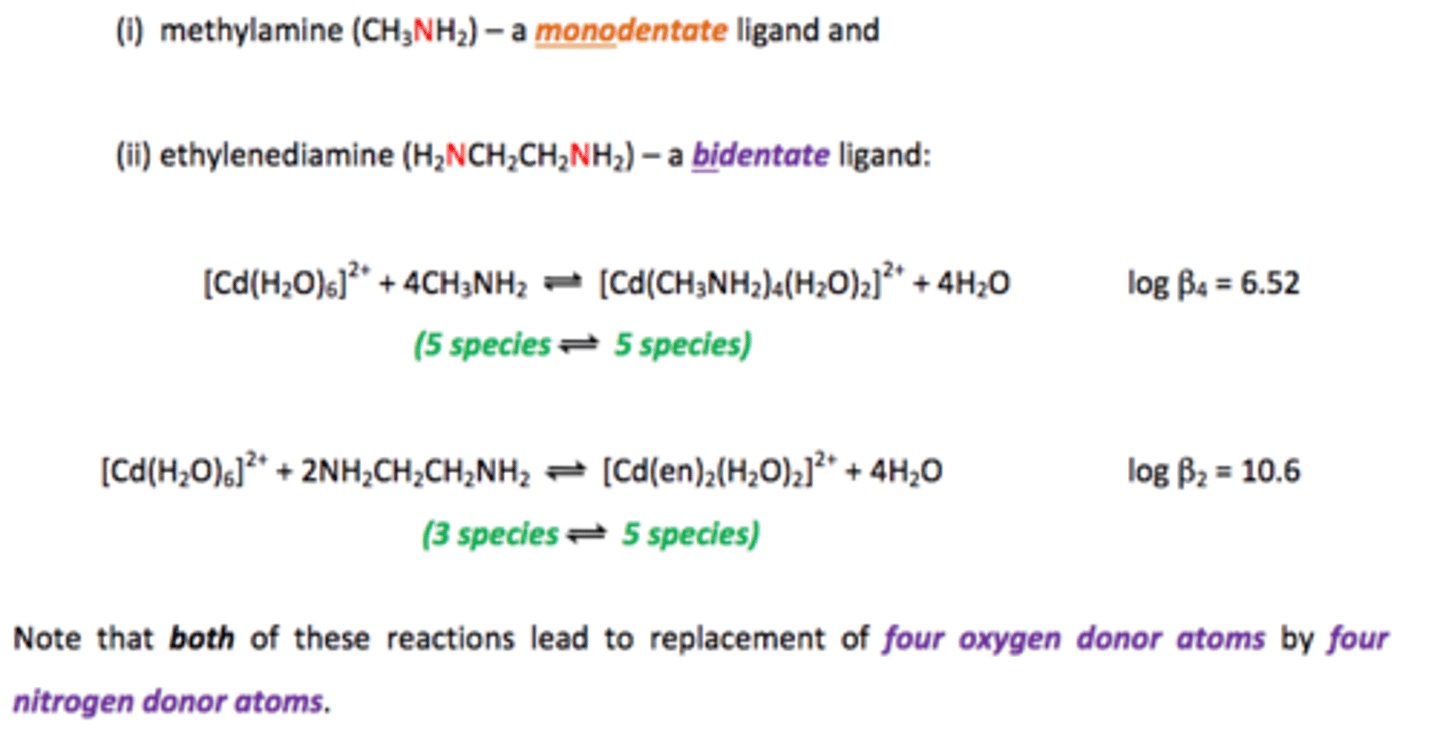
Why is it difficult to reverse reactions with Chelate effect?
Reversing would cause a decrease in entropy
Why do transition metals exist in variable oxidation states?
D orbital electrons have similar energies so multiple oxidation states are stable
Vanadium - oxidation states and colours
V²⁺ - violet
V³⁺ - green
VO²⁺ - blue
VO₂⁺ - yellow
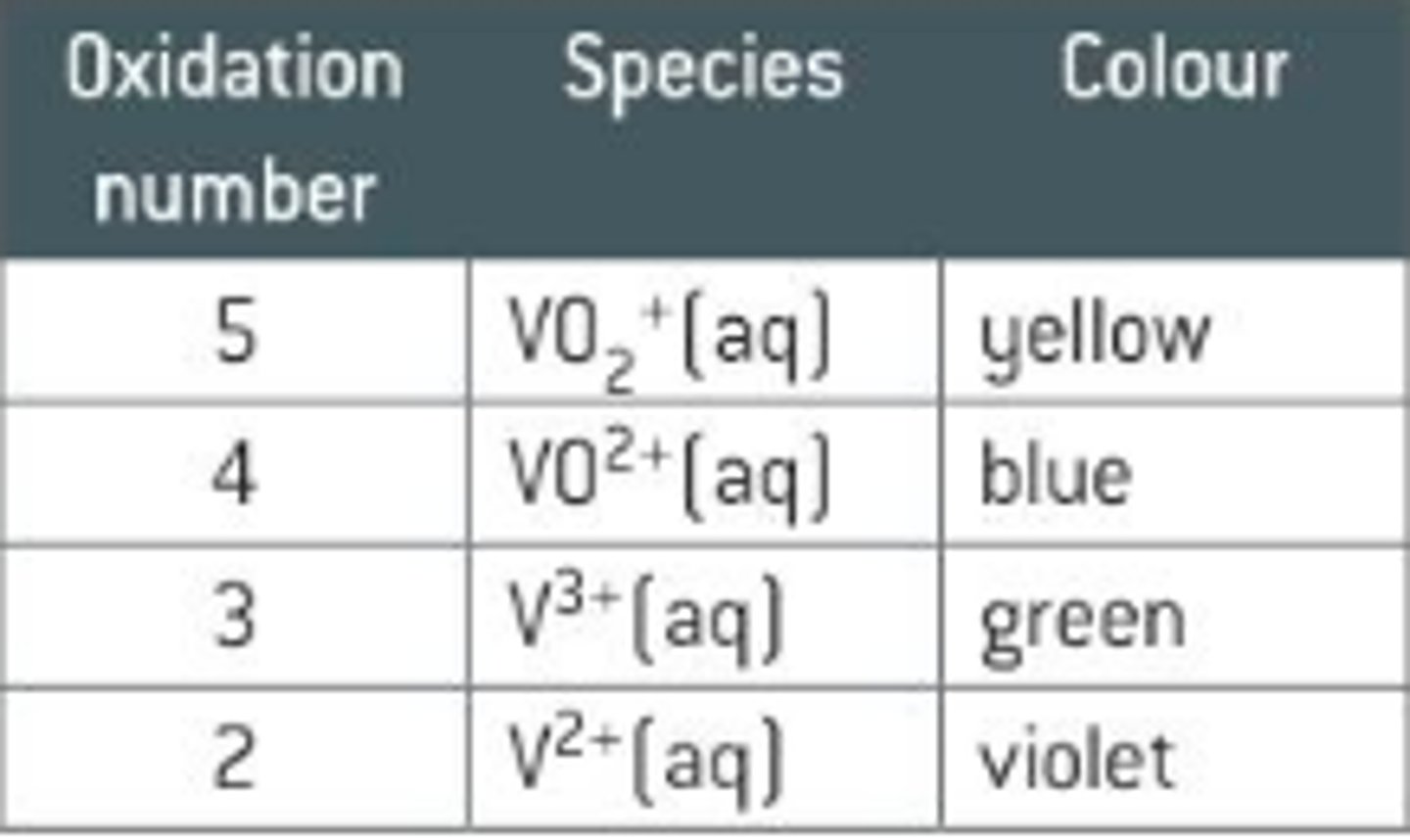
How are the variable oxidation states of V formed?
Reducing VO₂⁺ by zinc in ACIDIC SOLUTION
Equations showing reduction of VO₂⁺ to V²⁺
2VO₂⁺ + Zn + 4H⁺ -> 2VO²⁺ + Zn²⁺ + 2H₂O
(yellow -> blue)
2VO₂⁺ + Zn + 4H⁺ -> 2V³⁺ + Zn²⁺ + 2H₂O
(blue -> green)
2V³⁺ + Zn -> 2V²⁺ + Zn²⁺
(green -> violet)
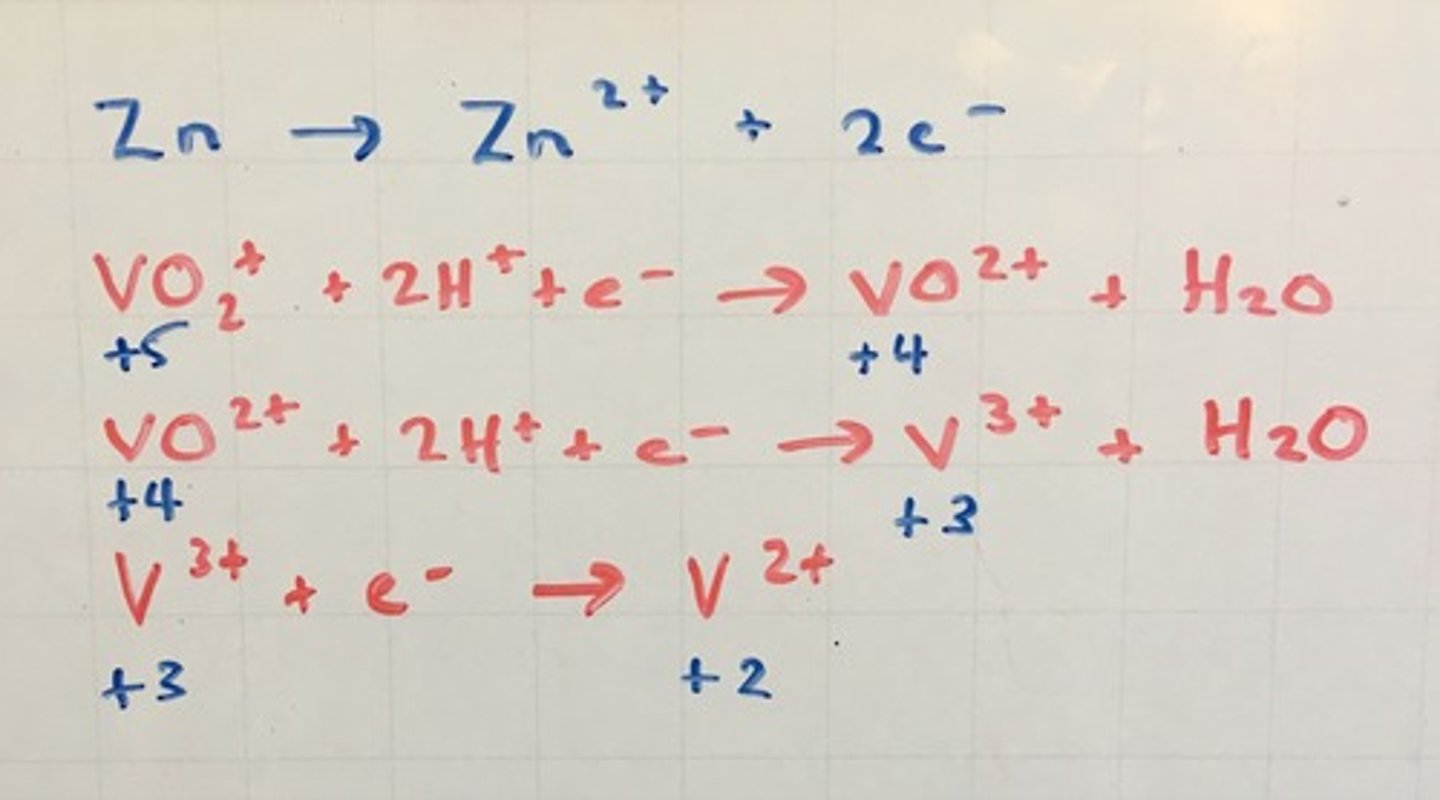
Redox Potential
How easily an atom/ion is reduced to a lower oxidation state (+ = easily reduced)
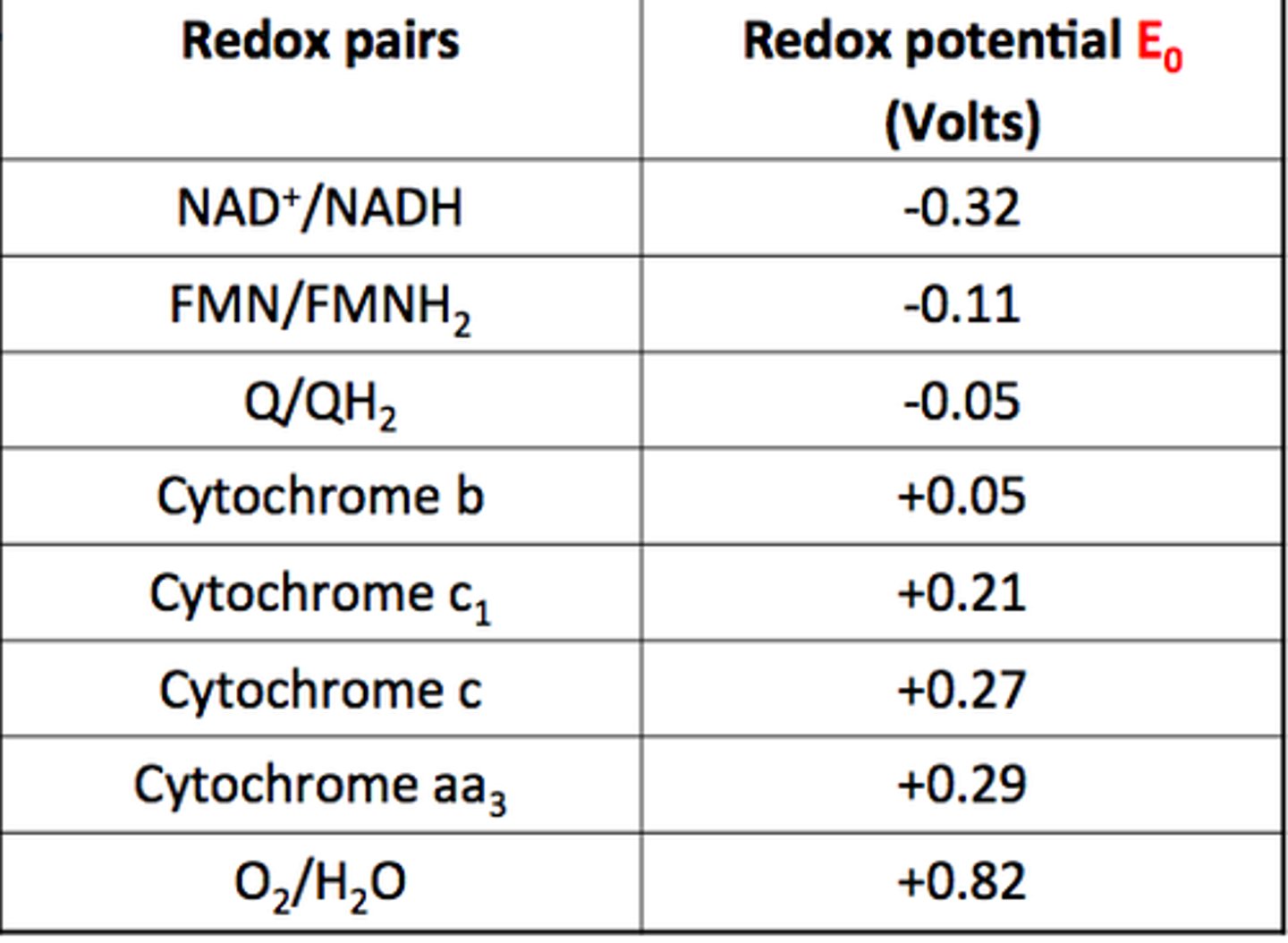
What 2 things is redox potential influenced by?
Ligands and pH
How do ligands affect redox potential?
E⦵ assumes ions are surrounded by water ligands in aqueous solution
- Other ligands that form stronger bonds with ion may change potential by stabilising a particular oxidation state
How does pH affect redox potential?
Some ions need H+ in order to be reduced (eg; V), others release OH- when reduced
What will redox potentials be in acidic conditions? Large or Small
Larger - ion is more easily reduced
Reduction of silver equation
Ag⁺ + e⁻ -> Ag (s)
(Electrode Potential = +0.80V - large so Ag can easily be reduced)
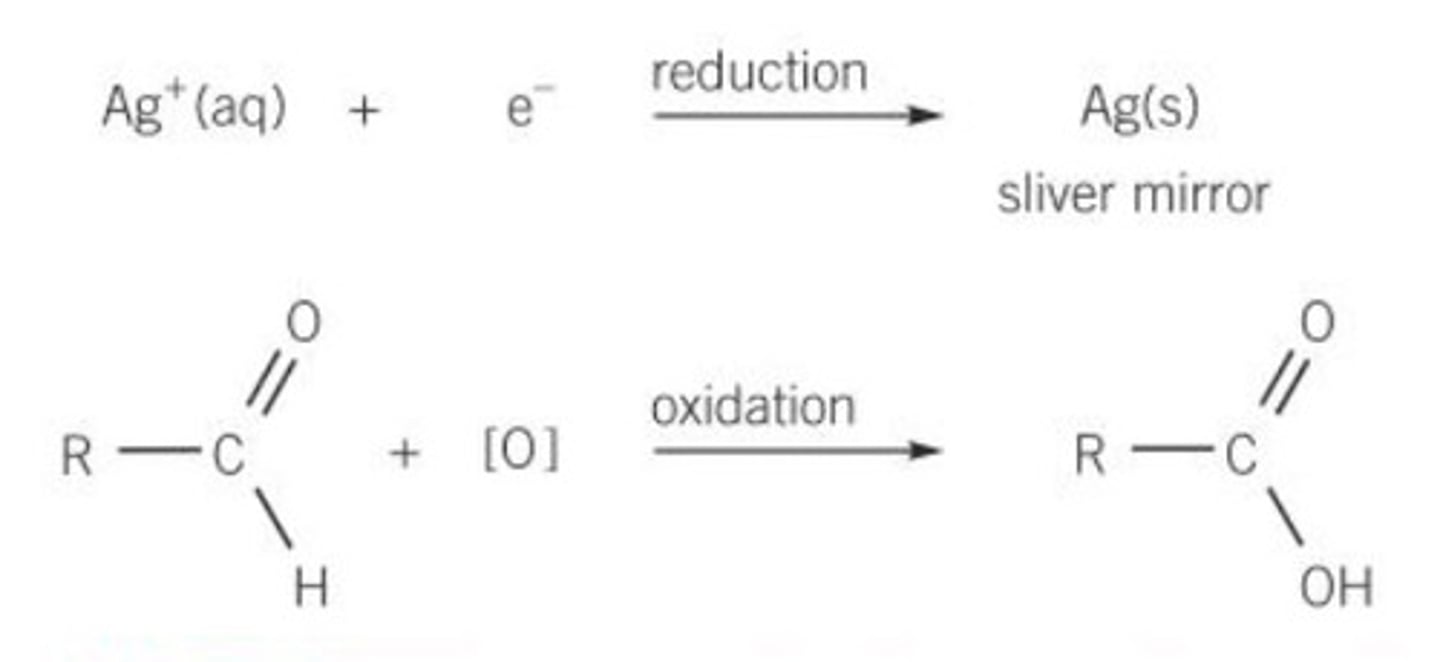
What is Tollens Reagent used for?
Distinguish between aldehydes and ketones (silver mirror for aldehydes)
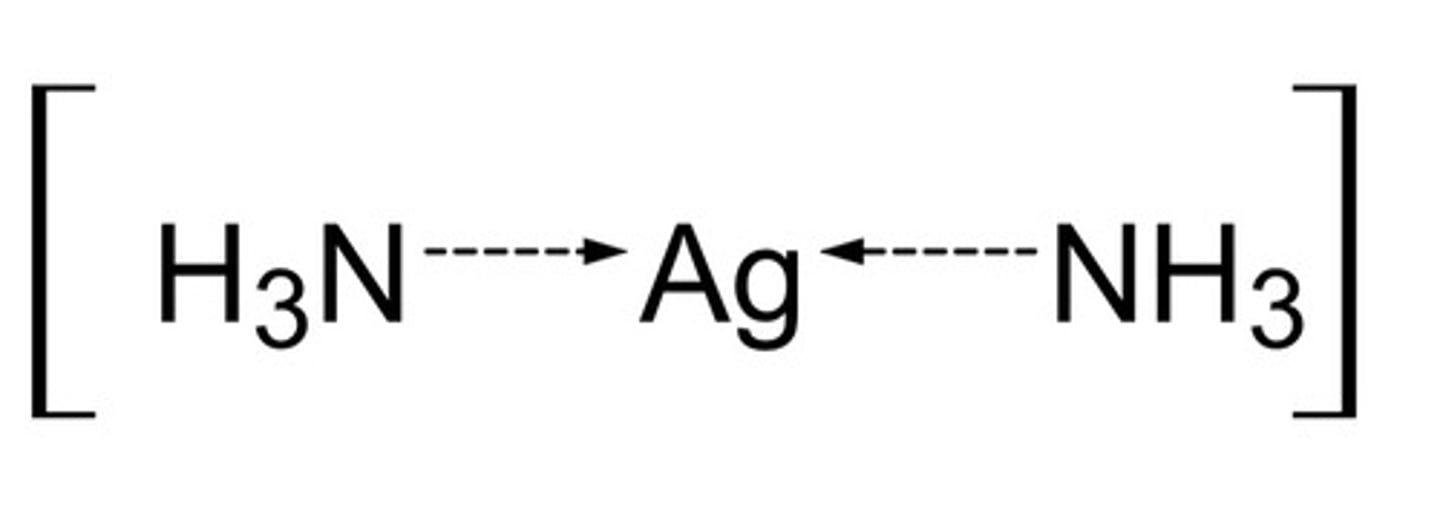
How is Tollen's reagent made?
React ammonia and silver nitrate to make [Ag(NH₃)₂]⁺
![<p>React ammonia and silver nitrate to make [Ag(NH₃)₂]⁺</p>](https://knowt-user-attachments.s3.amazonaws.com/c1a92c9f-fe44-4f84-a19b-c5c2082bd0e9.jpg)
What happens when Tollen's is added to an aldehyde?
- Aldehyde is oxidised to carboxylic acid
- Ag⁺ is reduced to Ag (silver mirror)

Equation for reaction of Tollens and Aldehyde
RCHO + 2[Ag(NH₃)₂]⁺ + 3OH⁻ --> RCOO⁻ + 2Ag + 4NH₃ + 2H₂O
Why are transition metals used in titrations?
- Variable oxidation states allows them to act as oxidising/reducing agents
- Colour change indicates endpoint

What happens in titrations with manganate ions (MnO₄⁻)?
Purple MnO₄⁻ ions are reduced to colourless Mn²⁺ ions by a reducing agent (eg; Fe²⁺ or C₂O₄²⁻)
Equation of reduction of MnO₄⁻ by Fe²⁺
MnO₄⁻ + 8H⁺+ 5Fe²⁺ -> Mn²⁺ +4H₂O + 5Fe³⁺
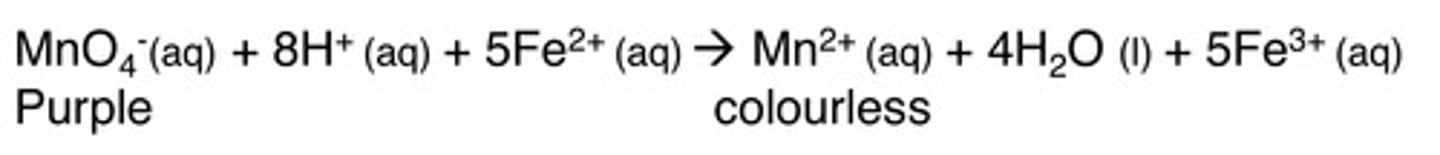
Why are transition metals good catalysts?
Variable oxidation states - multiple vacant d orbitals so easily receive/lose electrons

Heterogeneous Catalyst
Catalyst is in different state to the reactants (eg; solid catalysts for gaseous/liquid reactants)
How do heterogeneous catalysts work?
- Adsorb reactants onto active sites located on surface
- Bonds in reactants weaken and break
- Product desorbs
- Support mediums with high SA are used (eg; mesh/powder/pellets)
- High SA:Vol increases number of exposed active sites = lower activation energy
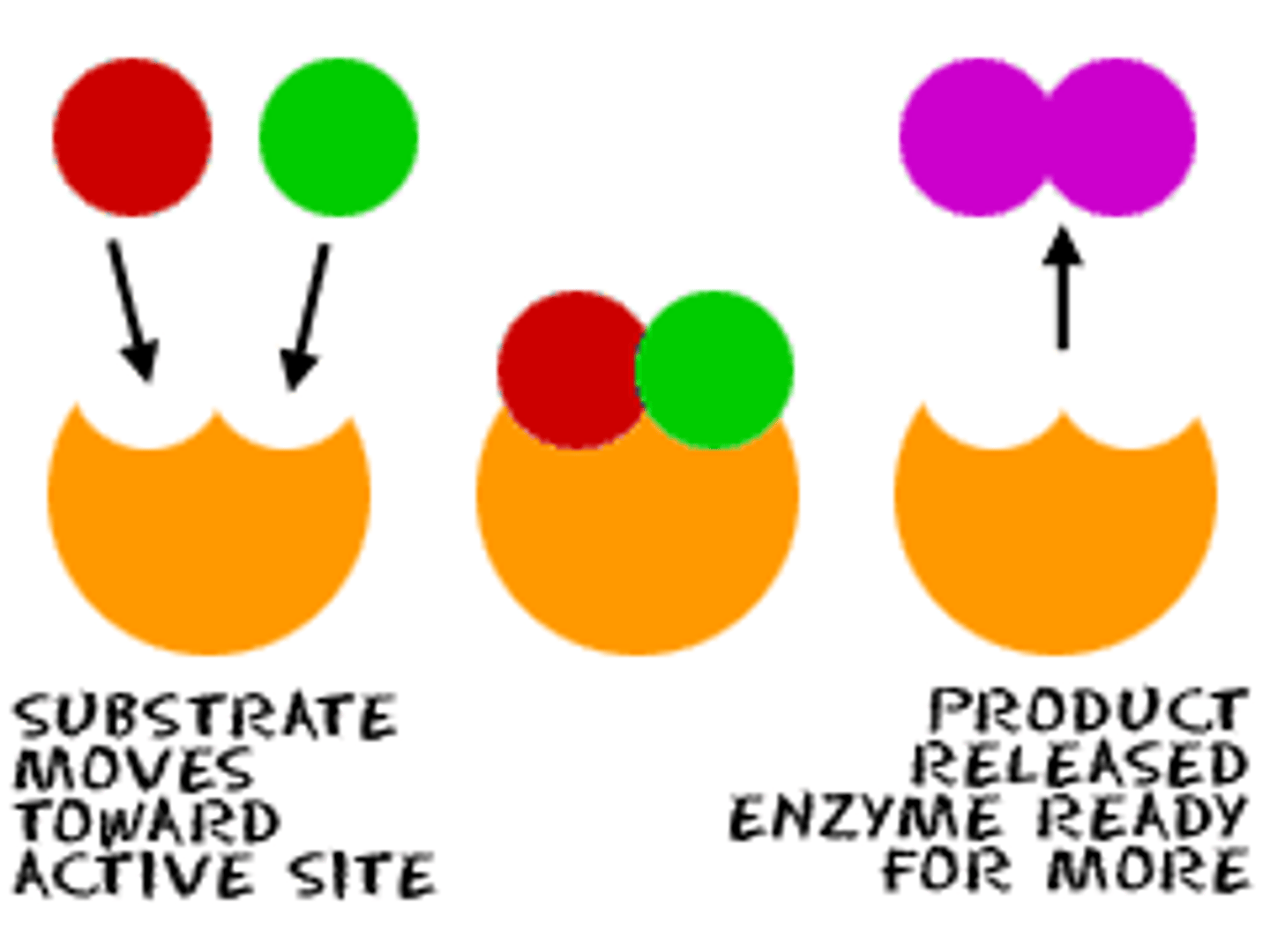
How does strength of adsorption affect catalyst?
- If adsorption is too strong = products can't be released
- If adsorption is too weak = not enough reactants adsorb
- Transition metals in the middle are the best
Examples of heterogeneous catalysts
- V₂O₅ when producing SO₃ in the Contact process
- Fe when producing NH₃ in the Haber process

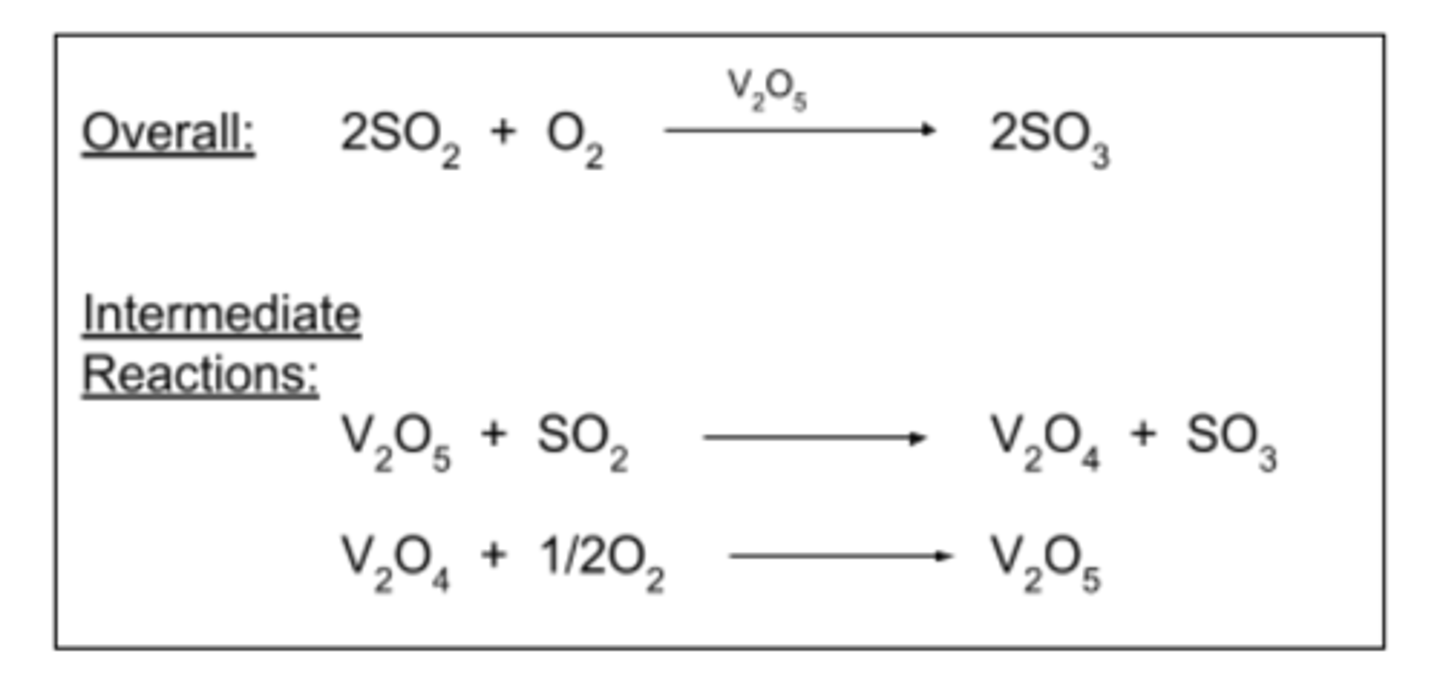
Contact Process - Equations
V₂O₅ + SO₂ -> V₂O₄ + SO₃
V₂O₄ + ½ O₂ -> V₂O₅
Overall: 2SO₂ + O₂ -> 2SO₃
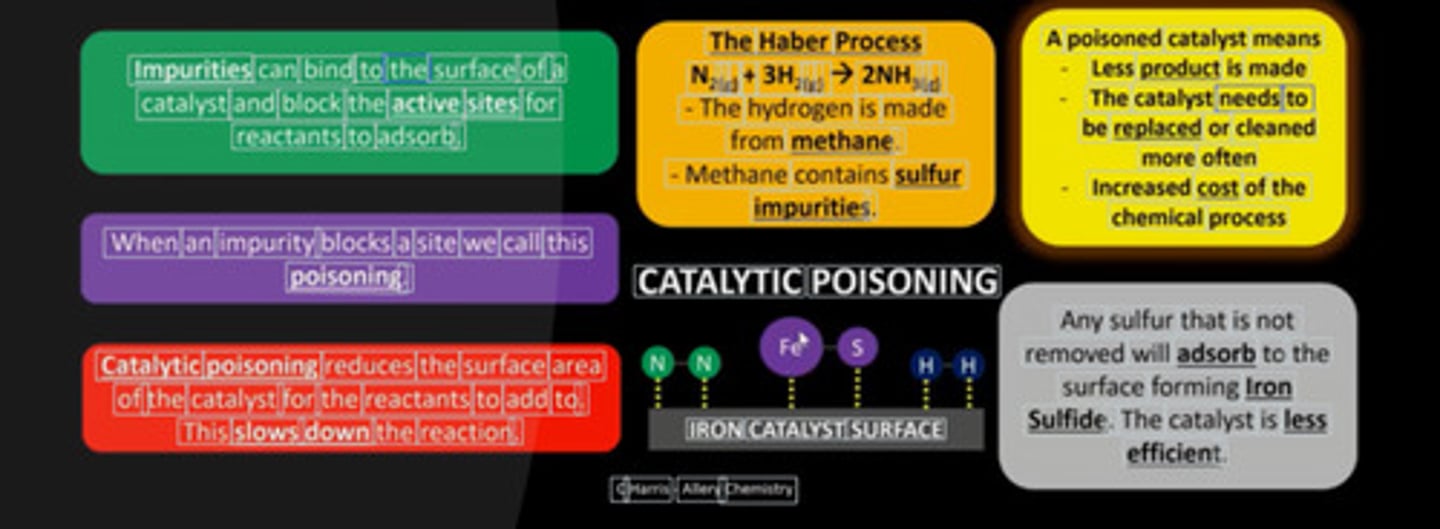
How can catalysts be poisoned?
- Impurities adsorb to surface and block active sites
- Reduces SA and reaction rate
- Increases operating costs due to less product
- Eg; sulfur (from H₂) poisons Fe in Haber process
Homogeneous catalysts
Catalyst is in the same phase as reactants
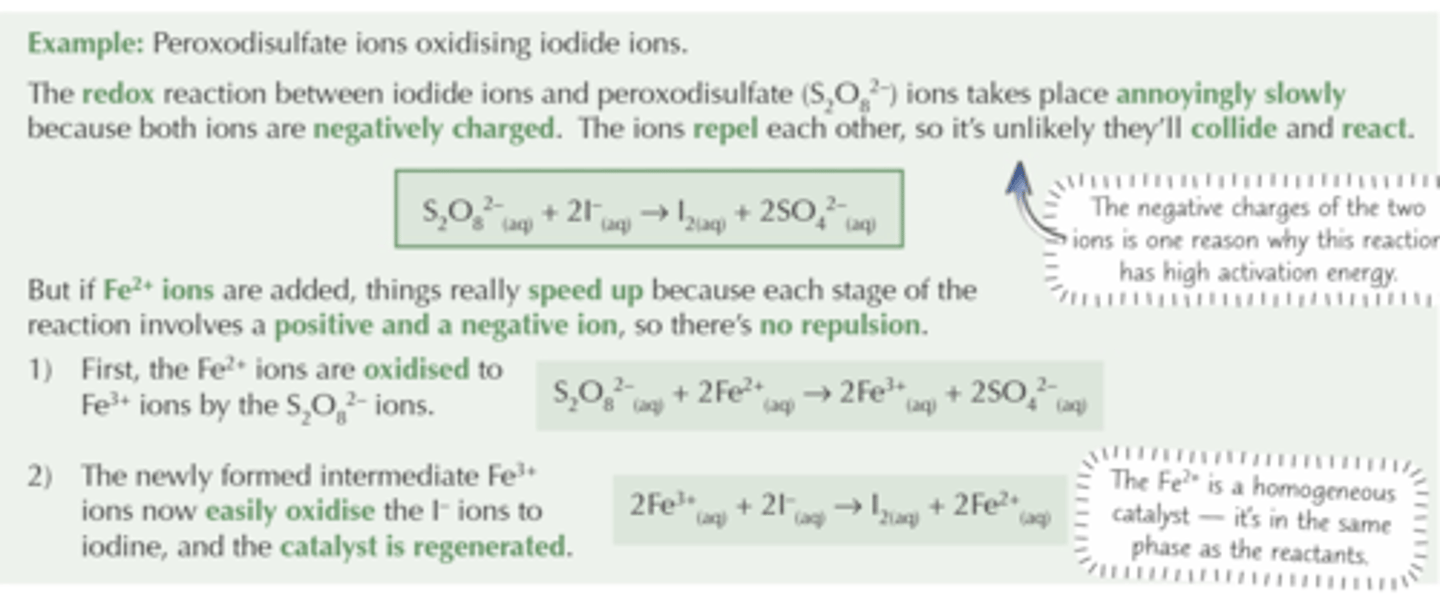
How do homogeneous catalysts work?
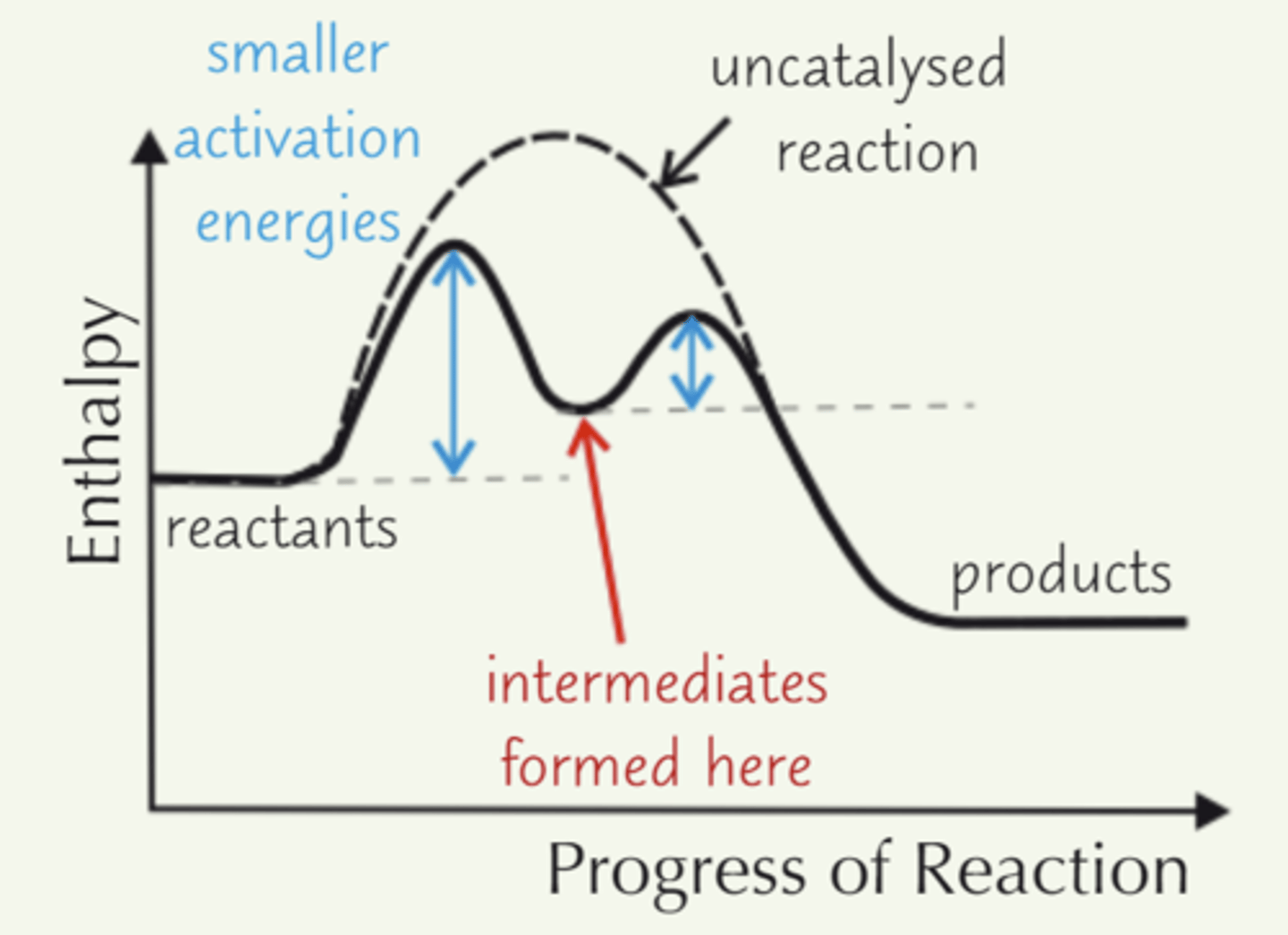
Form intermediates with reactants, which decompose to products - lowering AE
Examples of homogeneous catalysts
- Mn²⁺ ions catalyses MnO₄⁻ and C₂O₄²⁻
- Fe²⁺ catalyses S₂O₈²⁻ and I⁻
Role of Fe²⁺ in reaction between S₂O₈²⁻ and I⁻
- S₂O₈²⁻ and I⁻ react very slowly because they're both negative so repel each other (few collisions)
- Fe²⁺ has opposite charge to S₂O₈²⁻ so increases rate
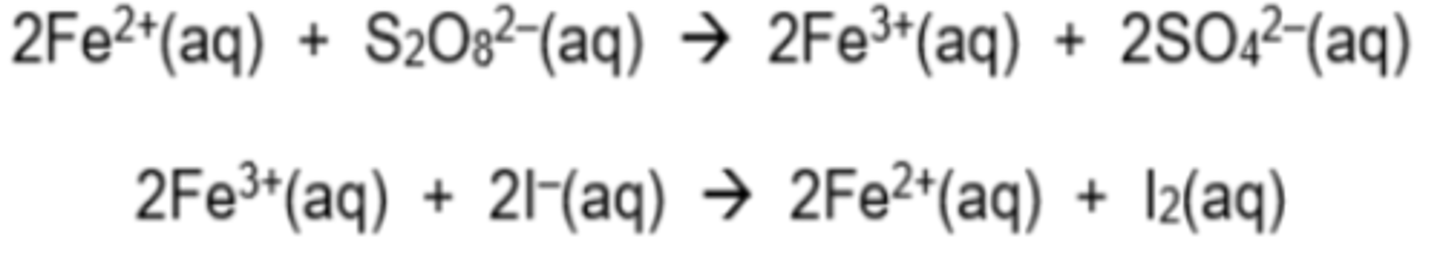
Equation for catalysed reaction of S₂O₈²⁻ and I⁻
S₂O₈²⁻ + 2Fe²⁺ ➔ 2Fe³⁺ + 2SO₄²⁻
2Fe³⁺ + 2I⁻ ➔ I₂ + 2Fe²⁺
Overall: S₂O₈²⁻ + I⁻ ➔ I₂ + 2SO₄²⁻
Autocatalysis of Mn²⁺ between MnO₄⁻ and C₂O₄²⁻
MnO₄⁻ + 4Mn²⁺ + 8H⁺➔ 5Mn³⁺ + 4H₂O
2Mn³⁺ + C₂O₄²⁻ ➔ 2Mn²⁺ + 2CO₂
Overall: 5C₂O₄²⁻ + 2MnO₄⁻ + 16H⁺ ➔ 10CO₂ + 2Mn²⁺ + 8H₂O
What must the electrode potential of a homogeneous catalyst be?
Electrode potential must lie in-between the electrode potentials of the 2 reactants
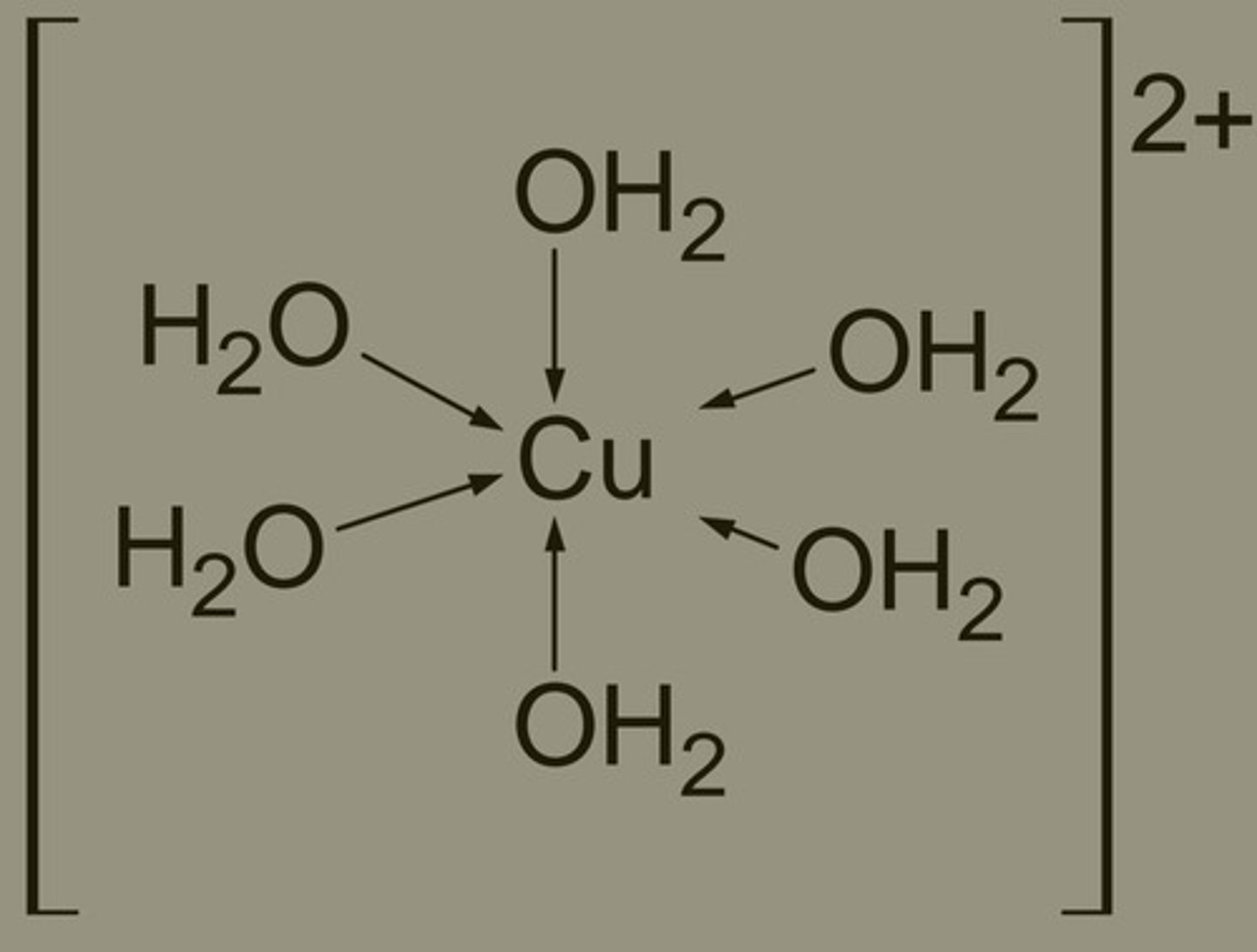
How do metal ions get hydrated?
• Transition metal dissolves in water
• Water molecules attach to metal ion via dative covalent bonds, forming metal-aqua complex ions - [M(H₂O)₆]ⁿ⁺
![<p>• Transition metal dissolves in water</p><p>• Water molecules attach to metal ion via dative covalent bonds, forming metal-aqua complex ions - [M(H₂O)₆]ⁿ⁺</p>](https://knowt-user-attachments.s3.amazonaws.com/dda29d10-08c8-4a6b-ad23-375c352159d1.jpg)
What is the pH of metal-aqua ion solutions?
Acidic - undergoes hydrolysis (an acid-base reaction) with water
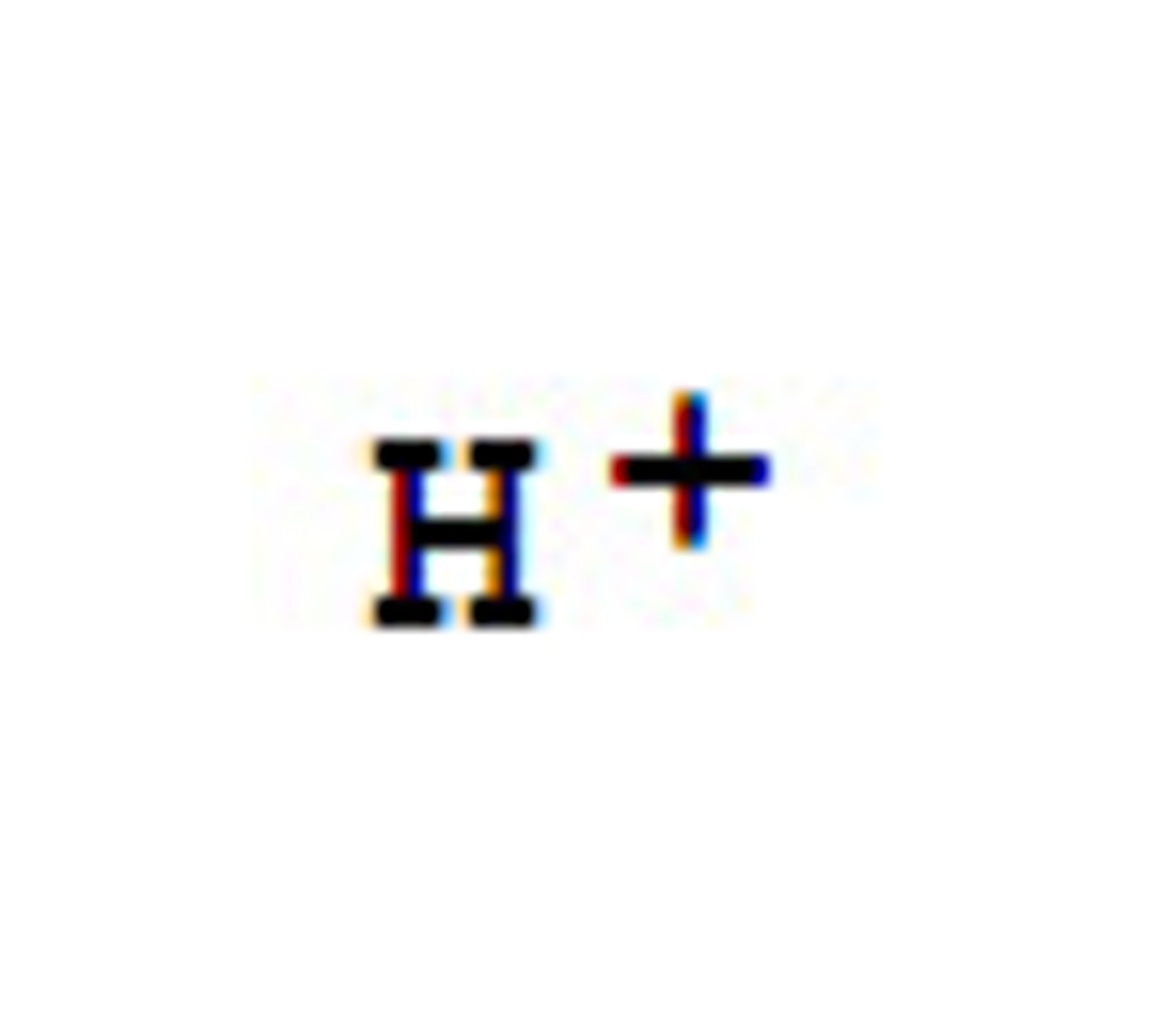
Why are 3+ ions more acidic than 2+ ions?
- 3+ ions have a greater charge density
- This polarises the water ligands more strongly
- This weakens the O-H bonds of H₂O
- H⁺ ions dissociate more easily
When do transition metal-aqua ions form precipitates?
- When mixed with NaOH, NH₃ or Na₂CO₃,
- Coloured precipitates are formed via ligand displacement reactions
Colours of the metals that make [M(H₂O)₆]²⁺
Fe (II) = green
Cu = blue

Colours of the metals that make [M(H₂O)₆]³⁺
Al = colourless
Fe (III) = violet (s) - appears yellow/brown in solution due to hydrolysis
Acidity/hydrolysis reaction of 2+ ions
[M(H₂O)₆]²⁺ + H₂O ⇌ [M(H₂O)₅(OH)]⁺ + H₃O⁺
Acidity/hydrolysis reaction of 3+ ions
[M(H₂O)₆]³⁺ + H₂O ⇌ [M(H₂O)₅(OH)]²⁺ + H₃O⁺
Reaction with OH- (2+ ions)
[Cu(H₂O)₆]²⁺ + 2OH⁻ --> Cu(H₂O)₄(OH)₂₍ₛ₎ + 2H₂O (l)
Colours of ppt in Reaction with OH-/NH₃ (2+ ions)
Cu = blue ppt
Fe (II) = green ppt
Reaction with OH- (3+ ions)
[Al(H₂O)₆]³⁺ + 3OH⁻ --> Al(H₂O)₃(OH)₃₍ₛ₎ + 3H₂O (l)
Colours of ppt in Reaction with OH-/NH₃ (3+ ions)
Al = white ppt
Fe(III) = brown ppt
Reaction with NH₃ (2+ ions)
[Cu(H₂O)₆]²⁺ + 2NH₃ --> Cu(H₂O)₄(OH)₂₍ₛ₎ + 2NH₄⁺(aq)
Reaction with NH₃ (3+ ions)
[Al(H₂O)₆]³⁺ + 3NH₃ --> Cu(H₂O)₃(OH)₃₍ₛ₎ + 3NH₄⁺(aq)
Amphoteric
Acts as both an acid and a base
What happens to Al(H₂O)₃(OH)₃ in excess NaOH?
White Al ppt dissolves to colourless solution
Al(H₂O)₃(OH)₃₍ₛ₎ + OH⁻ --> [Al(OH)₄]⁻ + 3H₂O
Al(H₂O)₃(OH)₃₍ₛ₎ + 3H⁺ --> [Al(H₂O)₆]³⁺(aq)
What happens to Cu(H₂O)₄(OH)₂ in excess NH₃?
Ligand substitution reaction - ppt dissolves to form deep blue solution
Cu(H₂O)₄(OH)₂₍ₛ₎ + 4NH₃ (aq) --> [Cu(NH₃)₄(H₂O)₂]²⁺(aq) + 2H₂O(l) + 2OH⁻(aq)
Reaction with CO₃²⁻ (2+ ions)
[Cu(H₂O)₆]²⁺ + CO₃²⁻ --> CuCO₃ + 6H₂O
Cu²⁺ + CO₃²⁻ --> CuCO₃
Colours of ppt in Reaction with CO₃²⁻ (2+ ions)
Cu = blue/green
Fe (II) = green
Reaction with CO₃²⁻ (3+ ions)
2[Fe(H₂O)₆]³⁺(aq) + 3CO₃²⁻(aq) --> 2Fe(OH)₃(H₂O)₃₍ₛ₎ + 3CO₂ + 3H₂O(l)
Colours of ppt in Reaction with CO₃²⁻ (3+ ions)
Al = white ppt
Fe (III) = brown ppt