ENTHALPY
0.0(0)
0.0(0)
Card Sorting
1/21
Earn XP
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
22 Terms
1
New cards
What does system mean in a chemical reaction?
the atoms and bonds involved in the reaction
2
New cards
Explain the law of conservation.
the amount of energy in an isolated system stays the same / energy cannot be created or destroyed, only transferred from one system to another
3
New cards
Breaking bonds is...?
endothermic
4
New cards
Making bonds is…?
exothermic
5
New cards
What is an endothermic reaction?
* reaction with an overall positive enthalpy change
* products > reactants
* products > reactants
6
New cards
What is an exothermic reaction?
* reaction with an overall negative enthalpy change
* reactants > products
* reactants > products
7
New cards
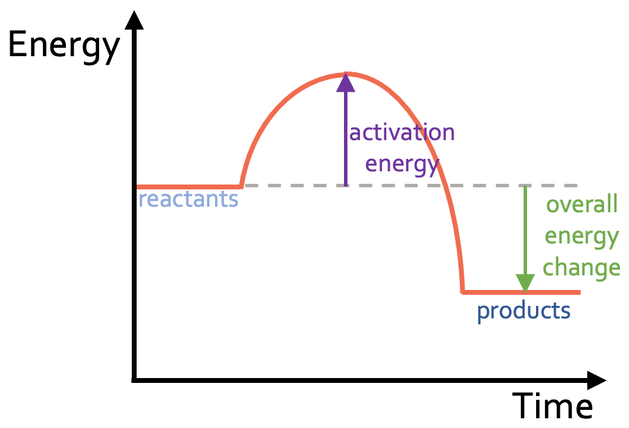
What type of reaction is shown in this enthalpy profile?
exothermic
8
New cards
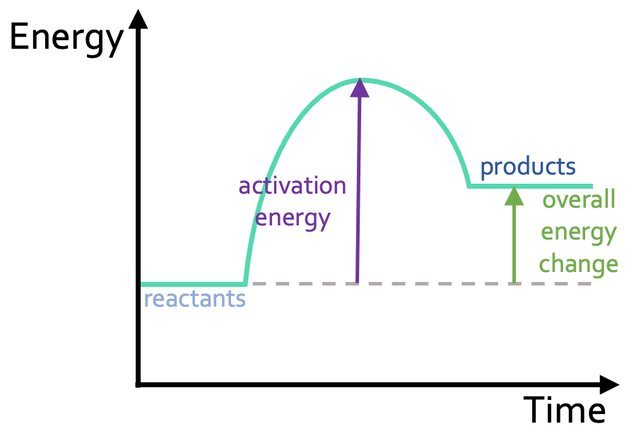
What type of reaction is shown in this enthalpy profile?
endothermic
9
New cards
What is activation energy (Ea)?
the minimum energy required for a reaction to take place
10
New cards
Which way does the arrow for Ea point in an enthalpy profile diagram?
always points upwards
11
New cards
What are the standard conditions?
* 101 kPa / 1atm
* 298K / 25°C
* 1 moldm⁻³
* 298K / 25°C
* 1 moldm⁻³
12
New cards
What does ‘standard state’ mean?
the state an element or compound exists at under standard conditions
13
New cards
What is standard enthalpy change of formation?
enthalpy change when 1 mol of a compound is formed from its constituent elements in their standard stated under standard conditions
14
New cards
What is standard enthalpy change of combustion?
enthalpy change when 1 mol of a substance reacts completely with oxygen with all reactants and products in their standard states
15
New cards
What is standard enthalpy change of neutralisation?
enthalpy change when 1 mol of water is formed in the reaction between an acid and a base, under standard conditions with all reactants and products in their standard states
16
New cards
What is standard enthalpy change of reaction?
enthalpy change accompanying a reacion in the molar quantities shown in the chemical equation in standard conditions with all reactants and products in their standard states
17
New cards
How can you calculate enthalpy change from experimental data?
Q = mc∆T
18
New cards
What are the advantages of using a bomb calorimeter?
* minimises heat loss
* pure oxygen used ensures complete combustion
* pure oxygen used ensures complete combustion
19
New cards
Why might experimental methods for enthalpy determination be inaccurate?
* heat lost to surroundings
* not under standard conditions
* reaction may not go to completion
* not under standard conditions
* reaction may not go to completion
20
New cards
What is average bond enthalpy?
the mean energy required to break 1 mol of bonds in gaseous molecules
21
New cards
How do you calculate enthalpy change of reaction using average bond enthalpies?
reactants - products (B.E. R-P)
22
New cards
Why are bond enthalpies less accurate than standard energy of combustion/formation?
enthalpies are a mean for the same bond in a different molecule whereas standard enthalpies are specific to the molecule