Chapter 16: Exothermic and Endothermic Reactions
16.1-Exothermic and Endothermic Reactions
Energy is moved around in chemical reactions
- Chemicals store a certain amount of energy, and different chemicals store different amounts
- If the products of a reactions store more energy than the original reactants, then they must have taken in the difference in energy between the products and reactants from the surroundings during the reaction
- But if they store less, then the excess energy was transferred to the surroundings during the reaction
In an exothermic reactions, heat is given out
An exothermic reaction is one which transfers energy to the surroundings, usually by heating
The best example of an exothermic reaction is burning fuels, also called combustion
This gives out a lot of energy, it’s very exothermic
Neutralisation reactions are also exothermic
Examples of exothermic reactions are self-heating cans and hand warmers
In an endothermic reaction, heat is taken in
An endothermic reaction is one which takes in energy from the surroundings, this is shown by a fall in temperature
They also have everyday uses such as:
- In some spots injury packs
- An example of an endothermic reaction is the reaction of citric acid with sodium hydrogen carbonate.
16.2-More Exothermic and Endothermic Reactions
Energy
- Chemicals store energy in their bonds. If the products of a reaction store more energy than the reactants, then energy must have been taken in from the surroundings.
- The overall amount of energy, however, doesn't change (if this is a closed system) - we know this is true, because energy cannot be created or destroyed
- When reactions happen in solution, if there are temperature changes that can be observed they can be measured using a thermometer.
- Reactions that take in more heat energy from their surroundings (than they give out) are called endothermic reactions. Examples include:
- citric acid and sodium hydrogen carbonate
- thermal decomposition
- Some sports injury packs are based on endothermic reactions.
- Reactions that release more heat energy to their surroundings (than they take in) are called exothermic reactions. Examples include:
- combustion
- many oxidation reactions
- neutralisation
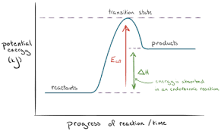
- Chemical reactions can be thought of as happening in two stages:
- Energy is needed to break the bonds in the reactants to form their separate atoms. This energy is called the activation energy, and is an endothermic process.
- The newly separated atoms form new bonds to make the products, and release energy to their surroundings. This is an exothermic process.
- The overall net movement of energy determines if the reaction is endothermic or exothermic. The reaction is:
- exothermic if more heat energy is released in forming bonds in the products than is required in breaking bonds in the reactants
- endothermic if less heat energy is released in forming bonds in the products than is required in breaking bonds in the reactants
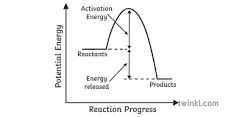
Reaction Profiles
- These are diagrams that show the relative energies of the reactants and products in a reaction - to show how energy changes over the course of a reaction.
- All reaction profiles start with an initial rise in energy, this is the activation energy and is the energy required for the reaction to begin.
- Whether the reaction then releases more (or less) energy than it initially required will determine if it is an exothermic or endothermic reaction.
Exothermic Reactions
- A reaction that releases energy can be shown on a reaction profile to initially have an increase (due to breaking bonds of reactants), but then has a bigger drop in energy overall (due to this energy being released from making new bonds).
Endothermic Reactions
- A reaction that takes in energy can be shown on a reaction profile to initially have an increase (due to breaking bonds of reactants), but then has a smaller drop in energy (due to bonds being made).
Practice Questions:
- A reaction that releases energy from the system in the form of heat is called an
- Exothermic reaction
- In which kind of reaction energy in the form of heat?
- Endothermic reactions
- Photosynthesis is popular example of which kind of reaction?
- Endothermic reaction
- What is the change in enthalpy in exothermic reaction?
- Negative