Radioactivity
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
What is the structure of an atom?
Atomic structure
Atoms are the building blocks of all matter
They are incredibly small, with a radius of only 1 × 10-10 m
Atoms have a tiny, dense nucleus at their centre, with electrons orbiting around the nucleus
The radius of the nucleus is over 10,000 times smaller than the whole atom, but it contains almost all of the mass of the atom
Atomic structure of lithium
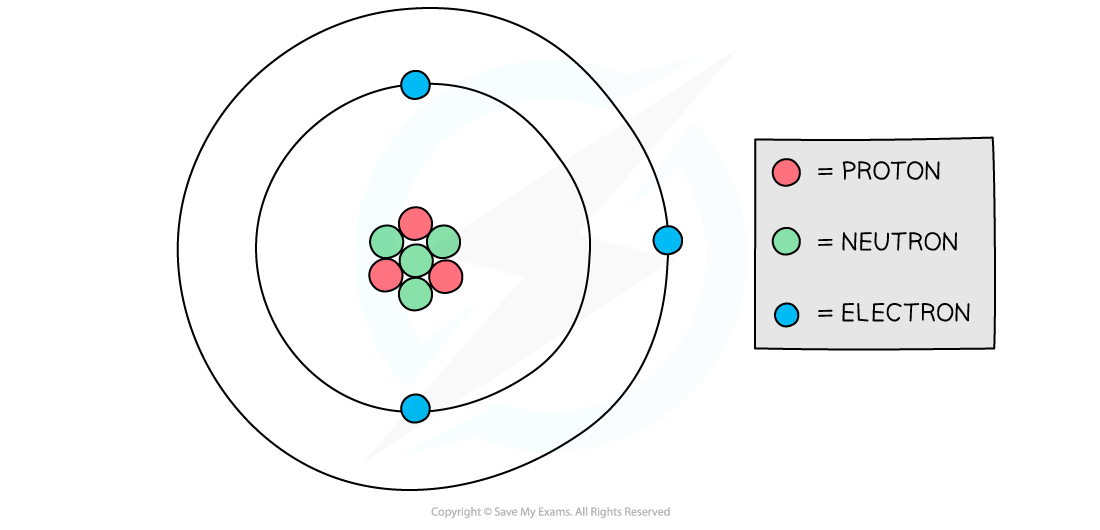
Diagram showing the structure of a Lithium atom. If drawn to scale then the electrons would be around 100 metres away from the nucleus!
Particles in the atom
The nucleus contains:
Protons - positively charged particles with a relative atomic mass of one unit
Neutrons – no charge, and also with a relative atomic mass of one unit
Almost all of the atom is empty space, but moving around the nucleus there are:
Electrons – negative charge with almost no mass (1/2000 the mass of a proton or neutron)
The properties of each of the particles are shown in the table below:
Table of particle properties
Particle | Location | Relative charge | Relative mass |
|---|---|---|---|
proton | in the nucleus | +1 | 1 |
neutron | in the nucleus | 0 | 1 |
electron | orbiting the nucleus | −1 | 1/2000 (negligible) |
Charge in the atom
Although atoms contain particles of different charge, the total charge within an atom is zero
This is because the number of electrons is equal to the number of protons
The following table sets out the calculation of the total charge in the lithium atom in the diagram above:
Calculating total charge table
Particle | Relative charge | Number of particles in lithium atom | number × relative charge | Total charge |
|---|---|---|---|---|
proton | +1 | 3 | +3 | (+3) + 0 + (−3) = 0 |
neutron | 0 | 4 | 0 | |
electron | −1 | 3 | −3 |
If an atom loses electrons, then it is said to be ionised
Symbols are used to describe particular nuclear by their element symbol, atomic number and mass number
This notation is called nuclear notation

Carbon 12 in nuclear notation
What is atomic and mass number?
Atomic & mass number
Atomic number
The number of protons in an atom is called its atomic number (it can also be called the proton number)
Elements in the periodic table are ordered by their atomic number
Therefore, the number of protons determines which element an atom is
The atomic number of a particular element is always the same
For example:
Hydrogen has an atomic number of 1. It always has just one proton
Sodium has an atomic number of 11. It has 11 protons
Uranium has an atomic number of 92. It has 92 protons
The atomic number is also equal to the number of electrons in an atom
This is because atoms have the same number of electrons and protons in order to have no overall charge
Mass number
The total number of particles in the nucleus of an atom is called its mass number
The mass number is the number of protons and neutrons in the atom
The number of neutrons can be found by subtracting the atomic number from the mass number
number of neutrons = mass number – atomic number
For example, if a sodium atom has a mass number of 23 and an atomic number of 11, then the number of neutrons would be 23 – 11 = 12
Nuclear notation
The mass number and atomic number of an atom are shown by writing them with the atomic symbol
This is called nuclear notation
Here are three examples:

Examples of nuclear notation for atoms of Hydrogen, Sodium and Uranium
The top number is the mass number
This is equal to the total number of particles (protons and neutrons) in the nucleus
The lower number is the atomic number
This is equal to the total number of protons in the nucleus
The atomic and mass number of each type of atom in the examples above is shown in this table:
Number of protons, neutrons & electrons table
Atom | Number of protons | Number of neutrons | Number of electrons |
|---|---|---|---|
hydrogen | 1 | 1 | 1 |
sodium | 11 | 12 | 11 |
uranium | 92 | 143 | 92 |
What is an isotope?
Isotopes
For a particular element, the number of protons is always the same, but the number of neutrons can be different
This is because the number of protons determines the element e.g. carbon atoms have 6 protons and iron atoms have 26 protons
An isotope is defined as:
An atom, or atoms, of the same element that have an equal number of protons but a different number of neutrons
Each element can have more than one isotope
Isotopes of hydrogen
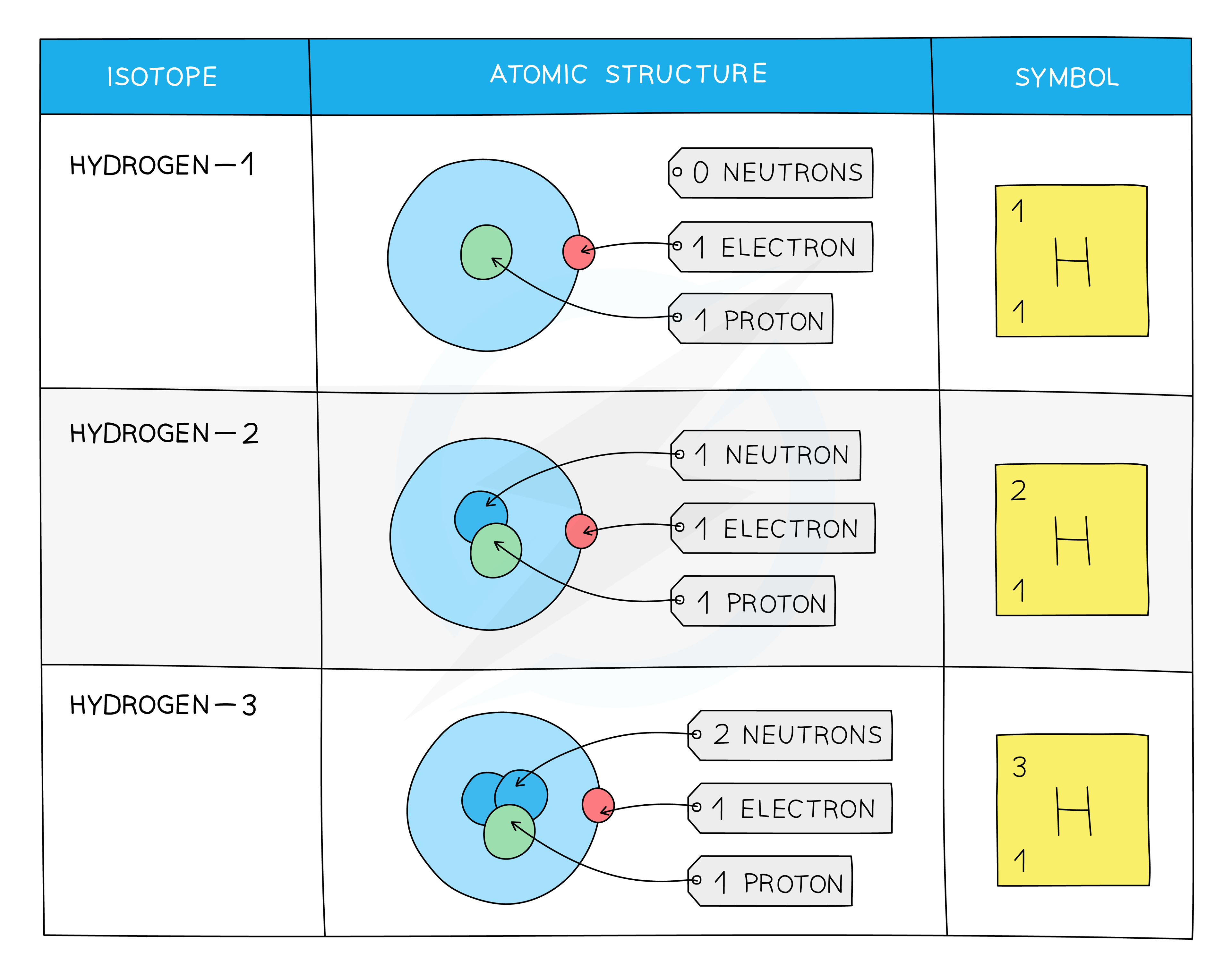
Some isotopes are more unstable than others due to the imbalance of protons and neutrons, which means
They may be more likely to decay
They may be less likely to occur naturally
For example, about 2 in every 10 000 atoms of hydrogen are the isotope deuterium
The isotope tritium is even rarer (about 1 in every billion billion atoms of hydrogen)
Why are particles radioactive and what are the different types of radiation?
Types of radiation
Some atomic nuclei are unstable and radioactive
This is because of an imbalance of protons or neutrons in the nucleus
Carbon-14 is an example of an isotope of carbon which is unstable
This is because it has two extra neutrons compared to a stable nucleus of carbon-12
Stable and unstable isotopes of carbon

Carbon-12 is stable, whereas carbon-14 is unstable because it has two extra neutrons
Unstable nuclei can emit radiation to become more stable
Radiation can be in the form of a high-energy particle or wave
This process is known as radioactive decay
As the radiation moves away from the nucleus, it takes some energy with it
This makes the nucleus more stable
Radioactive decay of a nucleus

Unstable nuclei decay by emitting high energy particles or waves
When an unstable nucleus decays, it emits radiation
The different types of radiation that can be emitted are:
Alpha (α) particles
Beta (β-) particles
Gamma (γ) radiation
These changes are spontaneous and random
What are the properties of the different types of radiation?
Properties of alpha, beta and gamma radiation
Alpha particles
The symbol for alpha is α
An alpha particle is the same as a helium nucleus
This is because it consists of two neutrons and two protons
Beta particles
The symbol for beta is β−
Beta particles are high-energy electrons
They are produced in nuclei when a neutron changes into a proton and an electron
Gamma rays
The symbol for gamma is γ
Gamma rays are electromagnetic waves
They have the highest energy of the different types of electromagnetic waves
Alpha, beta & gamma radiation

Alpha particles, beta particles and gamma waves can be emitted from unstable nuclei
Properties of alpha, beta & gamma
Alpha (α), beta (β) and gamma (γ) radiation can be identified by their:
Nature (what type of particle or radiation they are)
Ionising ability (how easily they ionise other atoms)
Penetrating power (how far can they travel before they are stopped completely)
Alpha, beta and gamma penetrate materials in different ways
This means they are stopped, or reduced, by different materials
Penetrating power of alpha, beta and gamma

Alpha, beta and gamma are different in how they penetrate materials. Alpha is the least penetrating, and gamma is the most penetrating
Alpha is stopped by paper, whereas beta and gamma pass through it
Beta is stopped by a few millimetres of aluminium
Gamma rays can pass through aluminium but are only partially stopped by thick lead
Summary of the properties of nuclear radiation
Particle | Nature | Range in air | Penetrating power | Ionising ability |
|---|---|---|---|---|
Alpha (α) | helium nucleus (2 protons, 2 neutrons) | a few cm | low; stopped by a thin sheet of paper | high |
Beta (β) | high-energy electron | a few 10s of cm | moderate; stopped by a few mm of aluminium foil or Perspex | moderate |
Gamma (γ) | electromagnetic wave | infinite | high; reduced by a few cm of lead | low |
How do you investigate the penetration powers of different types of radiation using either radioactive sources or simulations?
Aim of the experiment
The aim of this experiment is to investigate the penetration powers of different types of radiation using either radioactive sources or simulations
Variables:
Independent variable = Absorber material
Dependent variable = Count rate
Control variables:
Radioactive source
Distance of GM tube to source
Location / background radiation
Equipment List
Equipment | Purpose |
|---|---|
radioactive sources (α, β and γ) | to use as a source of radioactive emission |
ruler | to measure the distance between the source and detector |
mount for radioactive source | to secure the source in place |
Geiger-Muller tube and counter | to measure the count rate of a radioactive source |
tongs | to safely handle the sources at a distance |
selection of absorbing materials (paper, aluminium foil, lead) | to place between the source and detector to investigate effect on count rate |
lead-lined containers for radioactive sources | to store sources in when not in use |
Resolution of measuring equipment:
Ruler = 1 mm
Geiger-Müller tube = 0.01 μS/hr
Method

Apparatus for investigating the penetrating powers of different types of radiation
Connect the Geiger-Müller tube to the counter and, without any sources present, measure background radiation over a period of one minute
Repeat this three times, and take an average. Subtract this value from all subsequent readings.
Place a radioactive source a fixed distance of 3 cm away from the tube and take another reading of count rate over a period of one minute
Take a set of absorbers, i.e. some paper, several different thicknesses of aluminium (increasing in 0.5 mm intervals) and different thicknesses of lead
One at a time, place these absorbers between the source and the tube and take another reading of count rate over a period of one minute
Repeat the above experiment for other radioactive sources
Analysis of results
If the count rate is similar to background levels (allowing for a little random variation), then the radiation has all been absorbed
Note: some sources will emit more than one type of radiation
If the count rate reduces when paper is present, the source is emitting alpha
If the count rate reduces when a few mm of aluminium is present, then the source is emitting beta
If some radiation is still able to penetrate a few mm of lead, then the source is emitting gamma

Penetrating power of alpha, beta and gamma radiation
Evaluating the experiment
Systematic Errors:
Make sure that the sources are stored well away from the counter during the experiment
Conduct all runs of the experiment in the same location to avoid changes in background radiation levels
Random Errors:
The accuracy of such an experiment is improved with using reliable sources with a long half-life and an activity well above the natural background level
Safety considerations
When not using a source, keep it in a lead-lined container
When in use, try and keep a good distance (a metre or so) between yourself and the source
When handling the source, do so using tweezers (or tongs) and point the source away from you
Alpha, beta, gamma & neutron emission
Alpha decay
During alpha decay, an alpha particle is emitted from an unstable nucleus
A completely new element is formed in the process
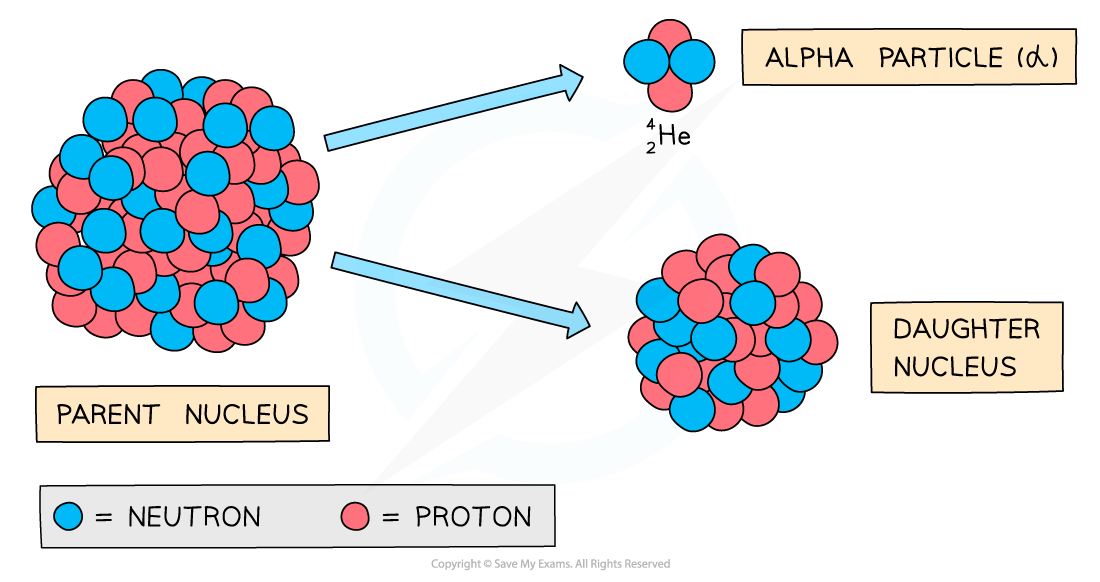
Alpha decay usually happens in large unstable nuclei, causing the overall mass and charge of the nucleus to decrease
An alpha particle is a helium nucleus
It is made of 2 protons and 2 neutrons
When the alpha particle is emitted from the unstable nucleus, the mass number and atomic number of the nucleus changes
The mass number decreases by 4
The atomic number decreases by 2
Alpha decay can be represented by the following nuclear equation:

Beta decay
During beta decay, a neutron changes into a proton and an electron
The electron is emitted and the proton remains in the nuclei
A completely new element is formed because the atomic number changes

Beta decay often happens in unstable nuclei that have too many neutrons. The mass number stays the same, but the atomic number increases by one
A beta particle is a high-speed electron
It has a mass number of 0
This is because the electron has a negligible mass, compared to neutrons and protons
Therefore, the mass number of the decaying nuclei remains the same
Electrons have an atomic number of -1
This means that the atomic number of the new nucleus will increase by 1 to balance the overall atomic number before and after the decay
Beta decay can be represented by the following nuclear equation:

Gamma decay
During gamma decay, a gamma ray is emitted from an unstable nucleus
This process makes the nucleus less energetic but does not change its structure

Gamma decay does not affect the mass number or the atomic number of the radioactive nucleus, but it does reduce the energy of the nucleus
The gamma ray that is emitted has a lot of energy, but no mass or charge
Gamma decay can be represented by the following nuclear equation:
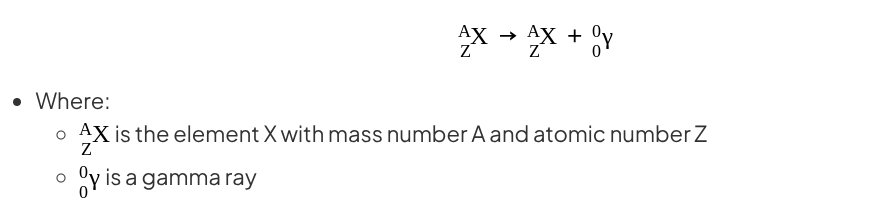
Notice that the mass number and atomic number of the unstable nucleus remains the same during the decay
Neutron emission
A small number of isotopes can decay by emitting neutrons
When a nucleus emits a neutron:
The atomic number (number of protons) does not change
The mass number (total number of nucleons) decreases by 1
Neutron emission can be represented by the following nuclear equation:
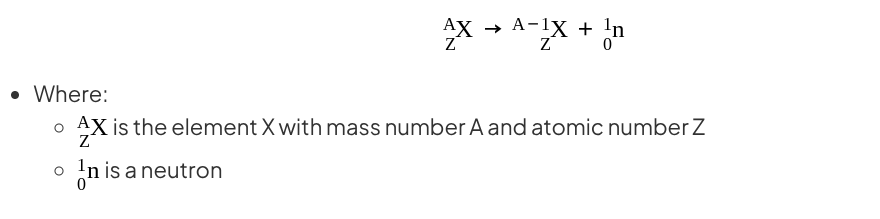
Notice that the atomic number remains the same during the decay but the mass number has changed
This means an isotope of the original element has formed
How an one detect ionising radiation?
Detecting radiation
Ionising radiation can be detected using
photographic film
a Geiger–Müller tube
Photographic film
Photographic films detect radiation by becoming darker when it absorbs radiation, similar to when it absorbs visible light
The more radiation the film absorbs, the darker it is when it is developed
People who work with radiation, such as radiographers, wear film badges which are checked regularly to monitor the levels of radiation absorbed
To get an accurate measure of the dose received, the badge contains different materials that the radiation must penetrate to reach the film
These materials may include aluminium, copper, paper, lead and plastic
The diagram shows what a typical radiation badge looks like:

A badge containing photographic film can be used to monitor a person’s exposure to radiation
Geiger-Müller tube
The Geiger-Müller tube is the most common device used to measure and detect radiation
Each time it absorbs radiation, it transmits an electrical pulse to a counting machine
This makes a clicking sound or displays the count rate
The greater the frequency of clicks, or the higher the count rate, the more radiation the Geiger-Müller tube is absorbing
Therefore, it matters how close the tube is to the radiation source
The further away from the source, the lower the count rate detected

A Geiger-Müller tube (or Geiger counter) is a common type of radiation detector
What is background radiation and what are the sources of it?
Background radiation
It is important to remember that radiation is a natural phenomenon
Radioactive elements have always existed on Earth and in outer space
However, human activity has added to the amount of radiation that humans are exposed to on Earth
Background radiation is defined as:
The radiation that exists around us all the time
Every second of the day there is some radiation emanating from natural sources such as:
Rocks
Cosmic rays from space
Foods
Chart of Background Radiation Sources

Background radiation is the radiation that is present all around in the environment. Radon gas is given off from some types of rock
There are two types of background radiation:
Natural sources
Artificial (man-made) sources
Natural Sources of Background Radiation
Radon gas from rocks and buildings
Airborne radon gas comes from rocks in the ground, as well as building materials e.g. stone and brick
This is due to the presence of radioactive elements, such as uranium, which occur naturally in small amounts in all rocks and soils
Uranium decays into radon gas, which is an alpha emitter
This is particularly dangerous if inhaled into the lungs in large quantities
Radon gas is tasteless, colourless and odourless so it can only be detected using a Geiger counter
Levels of radon gas are generally very low and are not a health concern, but they can vary significantly from place to place
Cosmic rays from space
The sun emits an enormous number of protons every second
Some of these enter the Earth’s atmosphere at high speeds
When they collide with molecules in the air, this leads to the production of gamma radiation
Other sources of cosmic rays are supernovae and other high energy cosmic events
Carbon-14 in biological material
All organic matter contains a tiny amount of carbon-14
Living plants and animals constantly replace the supply of carbon in their systems hence the amount of carbon-14 in the system stays almost constant
Radioactive material in food and drink
Naturally occurring radioactive elements can get into food and water since they are in contact with rocks and soil containing these elements
Some foods contain higher amounts such as potassium-40 in bananas
However, the amount of radioactive material is minuscule and is not a cause for concern
Artificial Sources of Background Radiation
Nuclear medicine
In medical settings, nuclear radiation is utilised all the time
For example, X-rays, CT scans, radioactive tracers, and radiation therapy all use radiation
Nuclear waste
While nuclear waste itself does not contribute much to background radiation, it can be dangerous for the people handling it
Nuclear fallout from nuclear weapons
Fallout is the residue radioactive material that is thrown into the air after a nuclear explosion, such as the bomb that exploded at Hiroshima
While the amount of fallout in the environment is presently very low, it would increase significantly in areas where nuclear weapons are tested
Nuclear accidents
Nuclear accidents, such as the incident at Chornobyl, contribute a large dose of radiation to the environment
While these accidents are now extremely rare, they can be catastrophic and render areas devastated for centuries
Accounting for background radiation
Background radiation must be accounted for when taking readings in a laboratory
This can be done by taking readings with no radioactive source present and then subtracting this from readings with the source present
This is known as the corrected count rate
Measuring background count rate

The background count rate can be measured using a Geiger-Müller (GM) tube with no source present
For example, if a Geiger counter records 24 counts in 1 minute when no source is present, the background radiation count rate would be:
24 counts per minute (cpm)
24/60 = 0.4 counts per second (cps)
Measuring the corrected count rate of a source

The corrected count rate can be determined by measuring the count rate of a source and subtracting the background count rate
Then, if the Geiger counter records, for example, 285 counts in 1 minute when a source is present, the corrected count rate would be:
285 − 24 = 261 counts per minute (cpm)
261/60 = 4.35 counts per second (cps)
When measuring count rates, the accuracy of results can be improved by:
Repeating readings and taking averages
Taking readings over a long period of time
How does the activity of a radioactive source change over a period of time, and how can it be measured?
Activity & decay
Objects containing radioactive nuclei are called sources of radiation
Sources of radiation decay at different rates which are defined by their activity
The activity of a radioactive source is defined as:
The rate at which the unstable nuclei decay
Activity is measured in becquerels
The symbol for Becquerels is Bq
1 Becquerel is equal to 1 nucleus in the source decaying in 1 second
How does activity vary with time?
The activity of a radioactive source decreases with time
This is because each decay event reduces the overall number of radioactive particles in the source
Radioactive decay is a random process
The randomness of radioactive decay can be observed by measuring the count rate of a source using a Geiger-Muller (GM) tube
When the count rate is plotted against time, fluctuations can be seen
These fluctuations provide evidence for the randomness of radioactive decay

The decreasing activity of a source can be shown on a graph against time. The fluctuations show the randomness of radioactive decay
What is a half-life?
Half life
It is impossible to know when a particular unstable nucleus will decay
It is possible to find out the rate at which the activity of a sample decreases
This is known as the half-life
Half-life is defined as:
The time it takes for the number of nuclei of a sample of radioactive isotopes to decrease by half
In other words, the time it takes for the activity of a sample to fall to half its original level
Different isotopes have different half-lives and half-lives can vary from a fraction of a second to billions of years in length
Measuring half life
To determine the half-life of a sample, the procedure is:
Measure the initial activity A0 of the sample
Determine the half-life of this original activity
Measure how the activity changes with time
The time taken for the activity to decrease to half its original value is the half-life
Half life calculationsHalf-life
Scientists can measure the half-lives of different isotopes accurately
Uranium-235 has a half-life of 704 million years
This means it would take 704 million years for the activity of a uranium-235 sample to decrease to half its original amount
Carbon-14 has a half-life of 5700 years
So after 5700 years, there would be 50% of the original amount of carbon-14 remaining
After two half-lives or 11 400 years, there would be just 25% of the carbon-14 remaining
With each half-life, the amount remaining decreases by half
A graph can be used to make half-life calculations
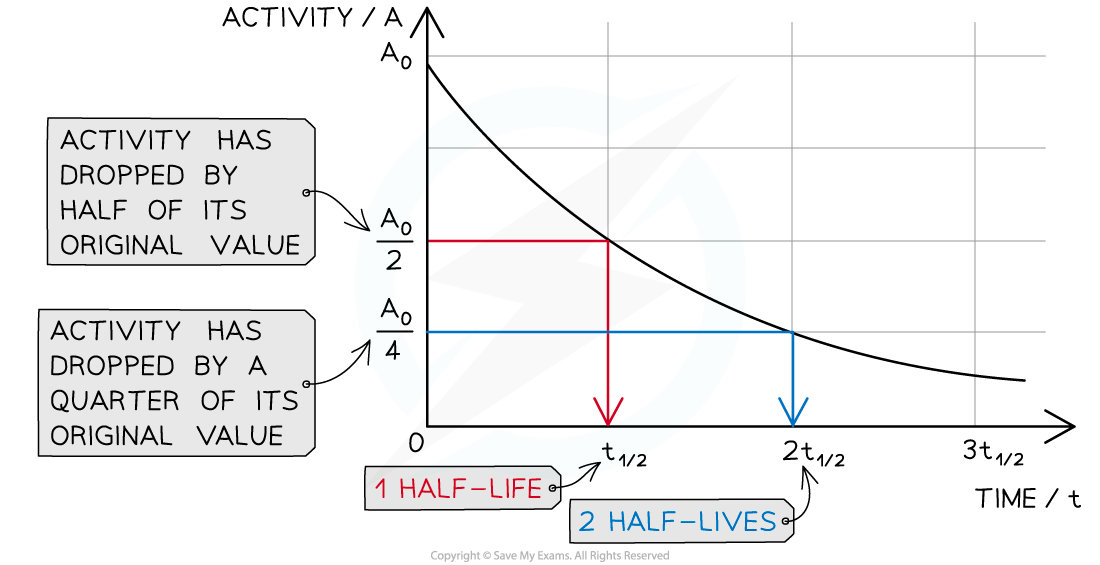
The graph shows how the activity of a radioactive sample changes over time. Each time the original activity halves, another half-life has passed
The time it takes for the activity of the sample to decrease from 100% to 50% is the half-life
It is the same length of time as it would take to decrease from 50% activity to 25% activity
The half-life is constant for a particular isotope
The following table shows that as the number of half-life increases, the proportion of the isotope remaining halves
Half life calculation tableHalf-life
number of half lives | proportion of isotope remaining |
|---|---|
0 | 100% |
1 | 50% |
2 | 25% |
3 | 12.5% |
4 | 6.25% |
What are the uses of radioactivity?
Uses of radioactivity
Radioactivity has many uses, such as:
Smoke detectors (alarms)
Monitoring the thickness of materials
Medical procedures including diagnosis and treatment of cancer
Sterilising food (irradiating food)
Sterilising medical equipment
Determining the age of ancient artefacts
The properties of the different types of radiation determine which one is used in a particular application
Smoke detectors
Alpha particles are used in smoke detectors
The alpha radiation will normally ionise the air within the detector, creating a current
The alpha emitter is blocked when smoke enters the detector
The alarm is triggered by a microchip when the sensor no longer detects alpha


When no smoke is present, alpha particles ionise the air and cause a current to flow. When smoke is present, alpha particles are absorbed and current is prevented from flowing which triggers the alarm
Measuring the thickness of materials
When a material, such as aluminium foil, moves above a beta source, some beta particles will be absorbed, but most will penetrate
The amount of beta particles passing through the material can be monitored using a detector
If the material gets thicker, more particles will be absorbed, and the count rate will decrease
If the material gets thinner, fewer particles will be absorbed, and the count rate will increase
This allows the manufacturer to make adjustments to keep the thickness of the material constant

Beta particles can be used to measure the thickness of thin materials such as paper, cardboard or aluminium foil
Beta radiation is used because the material will only partially absorb it
If an alpha source were used, all alpha particles would be absorbed regardless of material thickness
If a gamma source were used, almost all gamma rays would be detected regardless of material thickness
Diagnosis and treatment of cancer
Radiotherapy is the name given to the treatment of cancer using radiation
Note: this is different to chemotherapy which is a drug treatment for cancer
Although radiation can cause cancer, it is also highly effective at treating it
Ionising radiation can kill living cells
Some cells, such as bacteria and cancer cells, are more susceptible to radiation than others
Beams of gamma rays are directed at the cancerous tumour
Gamma rays are used as they can penetrate the body and reach the tumour
The beams are moved around to minimise harm to healthy tissue whilst still being aimed at the tumour
A tracer is a radioactive isotope that can be used to track the movement of substances, like blood, around the body
A PET scan can detect the emissions from a tracer to diagnose cancer and determine the location of a tumour

Radiation therapy is a type of cancer treatment which targets the tumour with ionising radiation
Sterilising food and medical equipment
Gamma radiation is widely used to sterilise medical equipment
Gamma is most suited to this because:
It is the most penetrating out of all the types of radiation
It is penetrating enough to irradiate all sides of the instruments
Instruments can be sterilised without removing the packaging
Food can be irradiated in order to kill any microorganisms that are present on it
This makes the food last longer and reduces the risk of food-borne infections

Food that has been irradiated carries this symbol, called the Radura. Different countries allow different foods to be irradiated
What is the difference between contamination and irradiation?
Contamination & irradiation
Contamination
Contamination is defined as:
The accidental transfer of a radioactive substance onto or into a material
A substance is only radioactive if it contains a source of ionising radiation
Contamination occurs when a radioactive isotope gets onto a material where it should not be
It is almost always a mistake or an accident e.g. a radiation leak
As a result of this, the small amounts of the isotope in the contaminated areas will emit radiation and the material becomes radioactive
Irradiation
Irradiation is defined as:
The process of exposing a material to ionising radiation
Irradiating a substance does not make it radioactive
However, it can kill living cells
Irradiation is usually a deliberate process, such as in the sterilisation of food or medical equipment
Surgical equipment is irradiated before being used in order to kill any micro-organisms on it before surgery
Food can be irradiated to kill any micro-organisms within it to make it last longer

This sign is the international symbol indicating the presence of a radioactive material
Protection from irradiation and contamination
Radiation can mutate DNA in cells and cause cancer through both irradiation and contamination
Therefore, it is important to reduce the risk of exposure to radiation
Contamination is particularly dangerous if a radioactive source gets inside the human body
For example, through the inhalation of radioactive gas particles, or ingesting contaminated food
The internal organs will be irradiated as the source emits radiation as it moves through the body
To prevent irradiation, shielding can be used to absorb radiation
Lead-lined suits are used to reduce irradiation for people working with radioactive materials
The lead absorbs most of the radiation that would otherwise hit the person
To prevent contamination, an airtight suit is worn by people working in an area where a radioactive source may be present
This prevents radioactive atoms from getting on or into the person

Lead shielding is used when a person is getting an x-ray, as well as for people who work with radiation. Contamination carries much greater risks than irradiation
Differences between irradiation and contamination
The differences between irradiation and contamination are summarised in the table below:
Comparison of irradiation and contamination table
| Irradiation | Contamination |
|---|---|---|
description | when an object is exposed to a source of radiation but does not become radioactive | when an object becomes radioactive due to the presence of a source of radiation |
source | exposure to source of radiation outside the object | exposure to source on or within the object |
prevention | blocked by using shielding such as lead | radiation cannot be blocked once an object is contaminated, but can be prevented by handling the source safely |
causes | caused by the deliberate exposure to radiation | caused by the accidental transfer of radioactive material |
What are the dangers of radiation?
Dangers of ionising radiation
All types of ionising radiation pose a danger if mishandled as they can
damage living cells and tissues
cause mutations which can lead to cancer
Effect of radiation on a living cell

Ionising radiation can cause damage to DNA. Sometimes the cell can successfully repair the DNA, but incorrect repairs can cause a mutation
Highly ionising types of radiation are more dangerous inside the body (if a radioactive source is somehow ingested)
Alpha sources are the most ionising, so they are likely to cause the most harm to living cells inside the body
Gamma sources are the least ionising (about 20 times lower than alpha particles), so they are likely to cause the least harm to living cells inside the body
Highly penetrating types of radiation are more dangerous outside the body
Gamma sources are the most penetrating, so they are able to pass through the skin and reach living cells in the body
Alpha sources are least penetrating, so they would be absorbed by the air before even reaching the skin
Safe handling of radioactive sources
The risks of radiation exposure can be minimised by
handing sources of radiation safely
monitoring exposure to radiation
To minimise the risks of contamination, safety practices must be followed, such as:
keeping radioactive sources in a shielded container when not in use, for example, a lead-lined box
wearing gloves and using tongs to handle radioactive materials
wearing protective clothing (particularly if the risk of inhalation or ingestion is high)
limiting the time that a radioactive source is outside of its container
To minimise the risks of irradiation to workers, it is important to monitor their exposure to radiation
To protect against over-exposure, the dose received by different activities is measured
A dosemeter measures the amount of radiation in particular areas and is often worn by radiographers, or anyone working with radiation
Badge for monitoring radiation exposure

A dosemeter, or radiation badge, can be worn by a person working with radiation in order to keep track of the amount of radiation they are receiving
Disposal of nuclear waste
Nuclear waste must be treated appropriately, depending on the type of radiation it emits
Alpha-emitting nuclear waste is easily stored in plastic or metal canisters
Beta-emitting nuclear waste is stored inside metal canisters and concrete silos
Gamma-emitting nuclear waste requires storage inside lead-lined, thick concrete silos
Radioactive waste of all types tends to emit dangerous levels of radiation for many years, so it must be stored securely for a very long time
Typically, waste with the highest levels of radioactivity must be buried underground in secure, geologically stable locations
Dealing with radioactive waste

Depending on the type of radiation emitted, nuclear waste is treated in different ways
Sources with long half-lives present a risk of contamination for a much longer time
Radioactive waste with a long half-life can be buried underground to prevent radioactive from being released into the environment
Radioactive waste must be stored in strong containers
The containers must be able to withstand harsh conditions over long periods
Containers must be designed to resist rust and corrosion
Rust-proof containers are often expensive and challenging to manufacture
The disposal site must have high security to prevent unauthorised access
The location of the disposal site must have a low risk of natural disasters, e.g. earthquakes
Carefully selecting the site and using strong containers will help prevent radioactive waste from leaking into groundwater
Radioactive waste can also be diluted in large volumes of seawater
This helps to minimise the concentration of radioactive materials