tutorial 3: Western Blot Day 2 SDS PAGE and electroblot
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
what is the running buffer made of?
Tris, glycine and SDS
what is the transfer buffer made of?
glycine, Tris, SDS and methanol
what is tris? what does it do?
tris(hydroxymethyl)aminomethane” and its conjugate acid is used in buffer solutions
(range of approx. 7-9 at room temperature)
what is PDVF?
“Polyvinylidene fluoride or polyvinylidene difluoride” and it is is a highly non-
reactive polymer that is used as the membrane material in western blots, where it immobilizs proteins, due to its non-specific affinity for amino acids
describe the pore size of a PDVF membrane
The pore size of the gel is very reproducible and is directly related to the ratio of acrylamide to Bis.
The resulting gels are described in terms of %T, the concentration (w/v) of acrylamide and Bis, and %C, the weight percentage of the cross-linker in T.
For proteins, %T values of 5–10% result in gels with relative molecular mass (Mr) ranges of 20 000–200 000 Da.
describe separation by SDS-polyacrylamide gel electrophoresis
The pore size of polyacrylamide gels, which are polymerized as the gel is formed, decreases as the concentration of the gel monomer (expressed as % T) increases.
However, it is also affected by the extent of cross-linkage: Increasing the concentration of cross-linking agent (bis) relative to the total monomer (expressed as % C) up to 5% by weight decreases the pore size.
Above 5% C, the pore size increases again because the cross-linking agent dimerizes with
Thus the size of the pores in the gel can be altered depending on the size of the proteins you want to separate:
The smaller the size of the protein of interest, the higher the percentage of acrylamide/bis.
The bigger the size of the protein of interest, the lower the percentage of acrylamide/bis
what does PAGE use?
uses a stacking gel and a running gel.
The stacking gel has a low concentration of acrylamide and the running gel a higher concentration capable of slowing the movement of the protein
generally how do proteins move in an electric field
determined by its net charge, its molecular radius and the magnitude of the applied field.
The net charge or molecular radius of natively folded proteins is not weight dependent.
so in their native state, different proteins with the same molecular weight would migrate at different speeds in an electrical field depending on their charge and 3D shape
why is SDS also used in the gel?
to make sure that once the proteins are linearized and negatively
charged throughout the run
describe a The Discontinuous Buffer System and the Stacking gel
To conduct the current from the cathode (negative) to the anode (positive) through the gel, a buffer is needed.
“Discontinuous” simply means that the buffer in the gel and the tank are
differentTypically, the system is set up with a stacking gel at pH 6.8, buffered by Tris-HCl, a running gel buffered to pH 8.8 by Tris-HCl and an electrode buffer at pH 8.3
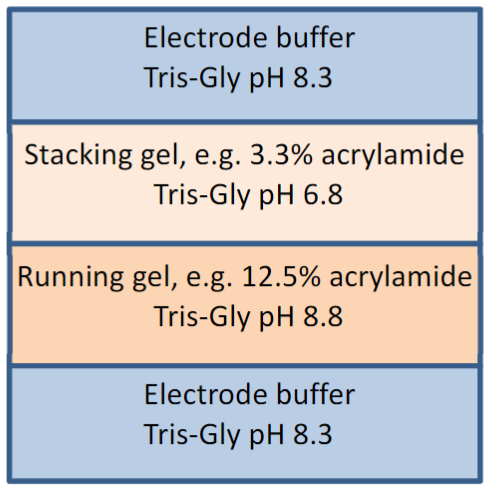
describe glycine’s states
can exist in three different charge states, positive, neutral or negative, depending
on the pH
When the power is turned on, the negatively-charged glycine ions in the pH 8.3 electrode buffer are forced to enter the stacking gel, where the pH is 6.8. In this environment, glycine switches predominantly to the neutrally charged state. This loss of charge causes them to move very slowly in the electric field.
describe the Cl- ion movement from Tris-HCl. how is this used to analyze proteins?
The Cl- ions (from Tris-HCl) move much more quickly in the electric field and they form an ion front that migrates ahead of the glycine. This results in two narrowly separated fronts of migrating ions; the highly mobile Cl- front, followed by the slower, mostly neutral glycine front
All of the proteins in the gel sample have a mobility that is intermediate between the extreme of the mobility of the glycine and Cl-, so when the two fronts sweep through the sample well, the proteins are concentrated into the narrow zone between the Cl- and glycine fronts
in the running gel, the pH switches to 8.8. what happens to the glycine molecules?
glycine molecules are mostly negatively charged and can migrate much faster than the proteins. So the glycine front accelerates past the proteins
describe running gel composition
Since the running gel has an increased acrylamide concentration, which slows the movement of the proteins according to their size, the separation begins.
Gel wells are around 1cm deep and you generally need to substantially fill them to get enough protein onto the gel.
So in the absence of a stacking gel, your sample would sit on top of the running gel, as a band of up to 1cm deep.
Rather than being lined up together and hitting the running gel together, this would mean that the proteins in your sample would all enter the
running gel at different times, resulting in very smeared bands: the stacking gel ensures that all of the proteins arrive at the running gel at the same time so proteins of the same molecular weight will migrate as tight bands.”
describe how coomassie blue stain works
Coomassie blue stain binds hydrophobically to the backbone of the protein
molecules. Therefore, Coomassie blue binding is nearly linear in binding to different proteins. So the dye can also interact electrostatically but noncovalently with the amino and carboxyl groups of proteins
describe how Coomassie works in acidic conditions
Coomassie gel stains and protein assay reagents are formulated as very acidic solutions in 25 to 50% methanol
In acidic conditions, the dye binds to proteins primarily through basic amino
acids (primarily arginine, lysine and histidine), and the number of coomassie dye ligands bound to each protein molecule is approximately proportional to the number of positive charges found on the protein.
explain the Electro-blot technique
Electrophoretic transfer is performed by placing the gel next to the membrane in a special cassette that, in turn, is placed in a tank of electrophoretic (transfer) buffer. The transfer buffer contains methanol.
“The presence of methanol in the transfer buffer serves two main purposes: It promotes dissociation of SDS from the protein and dramatically improves adsorption of proteins onto membranes in the presence of SDS, although these effects may vary with proteins.”
Upon application of voltage gradient perpendicular to the direction of the initial electrophoresis the gel, the sample migrates out of the gel and onto the filter paper; this is essentially the standard transfer methodology for western blot.
At the pH of the buffer (pH 8.3) most proteins are negatively charged and will migrate to the anode (positive electrode)e anode (positive electrode).”