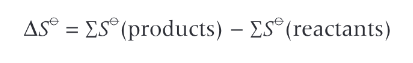22.4 entropy
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
general units of entropy
JK^-1mol^-1
general rules for the entropy value of solids, liquids and gases
solids have the smallest entropies
liquids have greater entropies
gases have the greatest entropies
when a substance changes from a solid to a liquid to a gas what happens to its entropy
it increases
melting and boiling increase the randomness of the particles
energy is spread out more and the entropy charge is positive
why do reactions that produce gases result in an increase in entropy
production of a gas increases the disorder of particles
energy is more spread out and the change in entropy is positive
standard entropy definition
the entropy of one mole of a substance, under standard condition (100kPa and 298K)
always have the unit of JK-1mol-1
are always positive
equation for calculating entropy changes