IB Physics Topic 7
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
electron volt (eV)
The Kinetic energy done to accelerate an electron through a potential difference of 1V.
internal energy
the sum of the kinetic and potential energies of all particles in the system
radioactive half-life
the time it takes for half of the radioactive isotopes in a sample to decay to a new form
isotopes
Atoms of the same number of protons but different numbers of neutrons
Nuecleon
all the particles inside the nucellus
nuclear binding energy
the energy required to decompose an atomic nucleus into its component protons and neutrons
alpha decay
radioactive decay by emission of an alpha particle
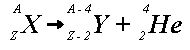
beta decay
A neutron changes into a proto to converse charge and emits an electron
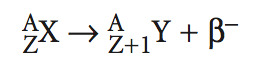
positron decay
When there are too many neutrons compared to protons, they emit a positron, which has the same mass as an electron.
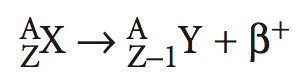
gamma decay
When a nucleus jumps into an energy state, it emits a photon
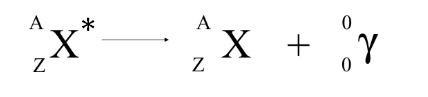
atomic mass units
A unit for describing the mass of an atom; 1 amu=1/12 the mass of a carbon-12 atom
Ohm's Law
the current in a circuit equals the voltage difference divided by the resistance